| 40 The Hypothalamus and Pituitary Gland
|
| The pituitary gland (also called the hypophysis) is a small (about 0.5 g in weight), yet complex endocrine structure at the base of the forebrain (Fig. 40-1). It is composed of an epithelial component called the adenohypophysis and a neural structure called the neurohypophysis. The adenohypophysis is composed of five cell types that secrete six hormones. The neurohypophysis releases several neurohormones. All endocrine functions of the pituitary gland are regulated by the hypothalamus and by negative- and positive-feedback loops.
|
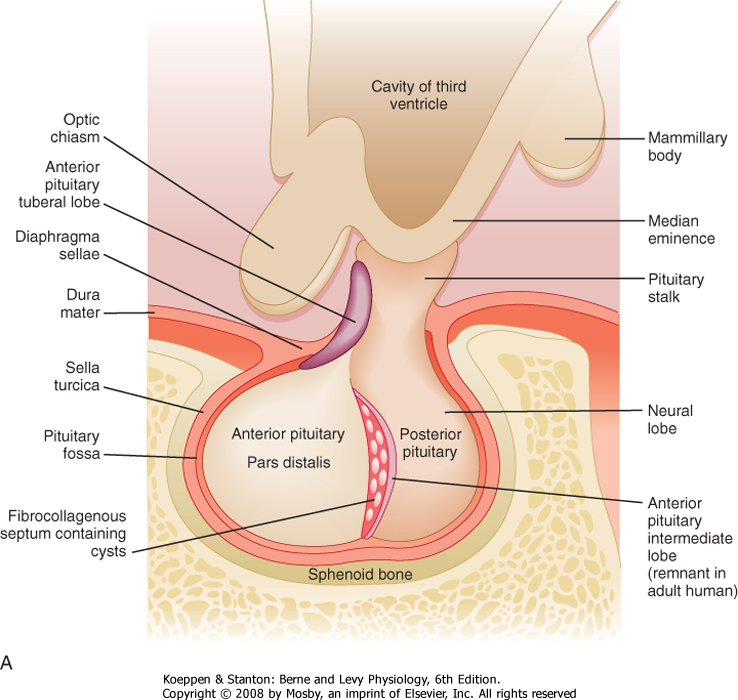
| Microscopic examination of the pituitary reveals two distinct types of tissue: epithelial and neural (Fig. 40-2). The epithelial portion of the human pituitary gland is called the adenohypophysis. The adenohypophysis makes up the anterior portion of the pituitary and is often referred to as the anterior lobe of the pituitary, and its hormones are referred to as anterior pituitary hormones. The adenohypophysis is composed of three parts: (1) the pars distalis, which makes up about 90% of the adenohypophysis; (2) the pars tuberalis, which wraps around the stalk; and (3) the pars intermedia, which regresses and is absent in adult humans.
|
| The neural portion of the pituitary is called the neurohypophysis and it represents a down-growth of the hypothalamus. The lowest portion of the neurohypophysis is called the pars nervosa, which is also called the posterior lobe of the pituitary (or simply, the "posterior pituitary"). At the superior end of the neurohypophysis, a funnel-shaped swelling called the median eminence develops. The rest of the neurohypophysis, which extends from the median eminence down to the pars nervosa, is called the infundibulum. The infundibulum and the pars tuberalis make up the pituitary stalk-a physical connection between the hypothalamus and the pituitary gland (Fig. 40-2).
|
| The pituitary gland (anterior and posterior lobes) is situated within a depression of the sphenoid bone called the sella turcica. Generally, cancers of the pituitary have only one way to expand, which is up into the brain and against the optic nerves. Thus, any increase in size of the pituitary is often associated with dizziness or vision problems, or both. The sella turcica is sealed off from the brain by a membrane called the diaphragma sellae.
|
| The pars nervosa is a neurovascular structure that is the site of release of neurohormones adjacent to a rich bed of fenestrated capillaries. The peptide hormones that are released are antidiuretic hormone (ADH, or arginine vasopressin) and oxytocin. The cell bodies of the neurons that project to the pars nervosa are located in the supraoptic nuclei (SON) and paraventricular nuclei (PVN) of the hypothalamus (in this context, a "nucleus" refers to a collection of neuronal cell bodies residing within the central nervous system [CNS]-a "ganglion" is a collection of neuronal cell bodies residing outside the CNS). The cell bodies of these neurons are described as magnocellular (i.e., large cell bodies), and they project axons down the infundibular stalk as the hypothalamohypophysial tracts. These axons terminate in the pars nervosa (Fig. 40-3). In addition to axonal processes and termini from the SON and PVN, there are glial-like supportive cells called pituicytes. The posterior pituitary is extensively vascularized and the capillaries are fenestrated, thereby facilitating diffusion of hormones into the vasculature.
|
| Synthesis of ADH and Oxytocin
|
| page 706 |  | | page 707 |
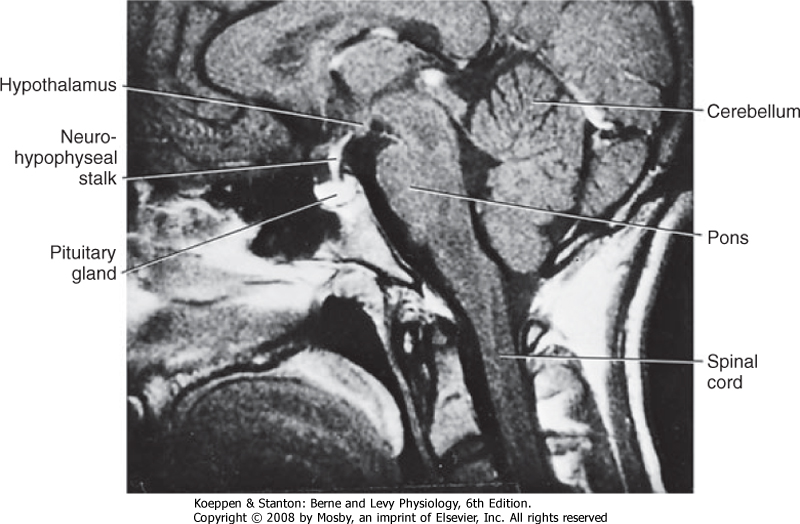
| Figure 40-1 Cross-sectional image of the head demonstrating the proximity of the hypothalamus and pituitary gland and their connection by a neurohypophyseal (pituitary) stalk. |
|
|
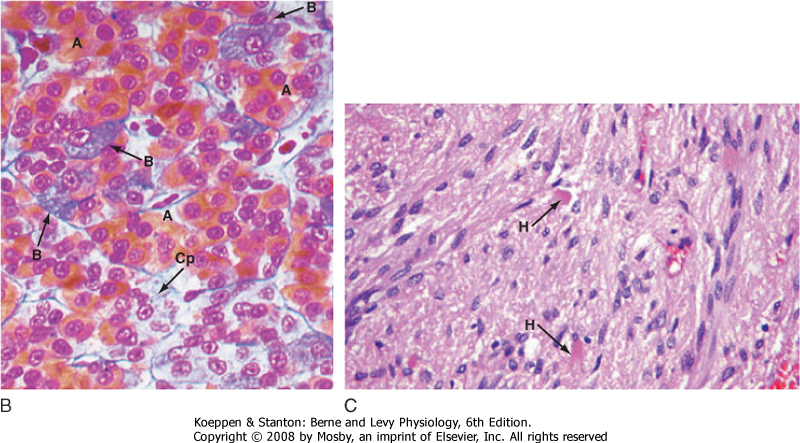
| Figure 40-2 A, Gross structure of the pituitary gland. The pituitary gland is below the hypothalamus and is connected to it by the pituitary stalk. The gland sits within the sella turcica, a fossa within the sphenoid bone, and is covered by a dural reflection, the diaphragma sellae. The pars distalis makes up most of the anterior pituitary. (Modified from Stevens A. In Lowe JS [ed]: Human Histology, 3rd ed. Philadelphia, Elsevier, 2005.) B, The pars distalis is derived from epithelial tissue that is composed of acidophils (A) (somatotropes and lactotropes) and basophils (B) (thyrotropes, gonadotropes, and corticotropes). The posterior pituitary is derived from neural tissue and has a histological appearance of nonmyelinated nerves (C). Cp, chromophobes; H, Herring bodies. (From Young B et al [eds]: Wheater's Functional Histology, 5th ed. Philadelphia, Churchill Livingstone, 2006.) |
| page 707 |  | | page 708 |
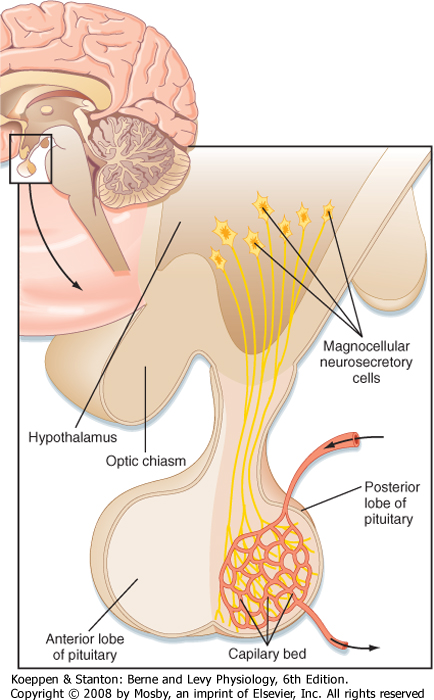
| Figure 40-3 Magnocellular neurons of the hypothalamus (paraventricular and supraoptic nuclei) project their axons down the infundibular process and terminate in the pars nervosa (posterior lobe), where they release their hormones (either ADH or oxytocin) into a capillary bed. (Modified from Larsen PR et al [eds]: Williams Textbook of Endocrinology, 10th ed. Philadelphia, Saunders, 2003.) |
| page 708 |  | | page 709 |
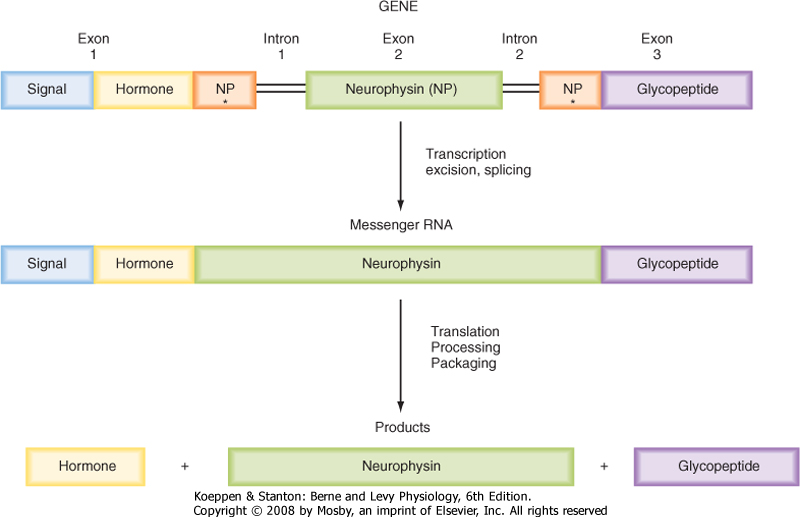
| Figure 40-4 Synthesis and processing of preprovasopressin or preprooxytocin. |
| ADH and oxytocin are nonapeptides (nine amino acids) that are similar in structure, differing in only two amino acids. They have limited overlapping activity. ADH and oxytocin are synthesized as preprohormones (Fig. 40-4). Each prohormone harbors the structure of oxytocin or ADH and a cosecreted peptide: either neurophysin I (associated with ADH) or neurophysin II (associated with oxytocin). These preprohormones are called preprovasophysin and preprooxyphysin. The N-signal peptide is cleaved as the peptide is transported into the endoplasmic reticulum. The prohormone is packaged in the endoplasmic reticulum and Golgi apparatus in a membrane-bound secretory granule in cell bodies within the SON and PVN (Fig. 40-5). The secretory granules are conveyed intraaxonally through a "fast" (i.e., millimeters per hour) ATP-dependent transport mechanism down the infundibular stalk to axonal termini in the pars nervosa. During
transit of the secretory granule, the prohormones are proteolytically cleaved to produce equimolar amounts of hormone and neurophysin. Secretory granules containing fully processed peptides are stored in the axonal termini. Axonal swelling because of the storage of secretory granule can be observed by light microscopy and is termed Herring bodies.
|
| ADH and oxytocin are released from the pars nervosa in response to stimuli that are primarily detected at the cell body and its dendrites in the SON and PVN of the hypothalamus. The stimuli are mainly in the form of neurotransmitters released from hypothalamic interneurons. With sufficient stimulus, the neurons will depolarize and propagate an action potential down the axon. At the axonal termini, the action potential increases intracellular [Ca++] and results in a stimulus-secretion response, with the exocytosis of ADH or oxytocin, along with neurophysins, into the extracellular fluid of the pars nervosa (Fig. 40-5). Hormones and neurophysins enter the peripheral circulation, and both can be measured in blood.
|
| Actions and Regulation of ADH and Oxytocin
|
| ADH primarily acts at the kidney to retain water (antidiuresis). The actions of ADH and regulation of ADH secretion were described in Chapter 34. Oxytocin primarily acts on the pregnant uterus (labor inducing) and myoepithelial cells of the breast (milk let-down during nursing). The actions and regulation of oxytocin are discussed in Chapter 43.
|
| Because posterior pituitary hormones are synthesized in the hypothalamus rather than the pituitary, hypophysectomy (pituitary removal) does not necessarily permanently disrupt synthesis and secretion of these hormones. Immediately after hypophysectomy, secretion of the hormones decreases. However, over a period of weeks, the severed proximal end of the tract will show histological modification and pituicytes will form around the neuron terminals. Secretory vacuoles are seen, and secretion of hormone resumes from this proximal end. Secretion of hormone can even potentially return to normal levels. In contrast, a lesion higher up on the pituitary stalk can lead to loss of neuronal cell bodies in the PVN and SON. |
 |
| The pars distalis is composed of five endocrine cell types that produce six hormones (Table 40-1). Because of the histological characteristics of the cell types, the corticotropes, thyrotropes, and gonadotropes are referred to as pituitary basophils, whereas the somatotropes and lactotropes are referred to as pituitary acidophils (Fig. 40-2, B).
|
| page 709 |  | | page 710 |
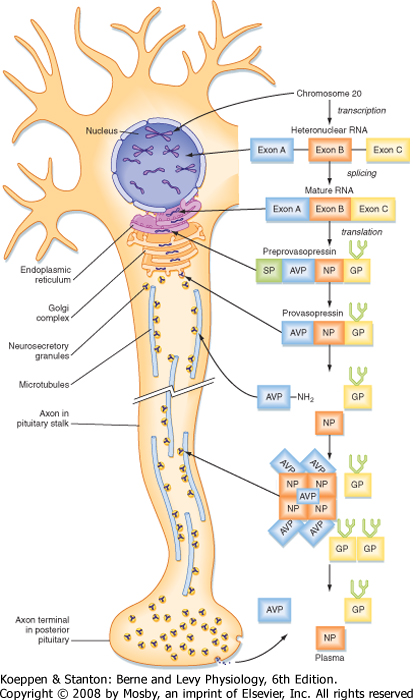
| Figure 40-5 Synthesis, processing, and transport of preprovasopressin. Human ADH (also called arginine vasopressin or AVP) is synthesized in the hypothalamic magnocellular cell bodies and packaged into neurosecretory granules. During intraaxonal transport of the granules down the infundibular process to the pars nervosa, provasopressin is proteolytically cleaved into the active hormone (AVP = ADH), neurophysin (NP), and a C-terminal glycoprotein (GP). NP arranges into tetramers that bind five AVP molecules. All three fragments are secreted from axonal termini in the pars nervosa (posterior pituitary) and enter the systemic blood. Only AVP (ADH) is biologically active. (Modified from Larsen PR et al [eds]: Williams Textbook of Endocrinology, 10th ed. Philadelphia, Saunders, 2003.) |
| page 710 |  | | page 711 |
| Significant progress has been made in understanding the differentiation of the five endocrine cells of the pars distalis from one precursor cell. The homeodomain transcription factor Prop-1 is expressed soon after Rathke's pouch forms and promotes the cell lineages of somatotropes, lactotropes, thyrotropes, and gonadotropes. In humans, rare mutations in the Prop-1 gene result in a type of combined pituitary hormone deficiency. These individuals display dwarfism because of lack of GH, mental retardation secondary to hypothyroidism, and infertility as a result of a lack of gonadotropins. A subsequently expressed, pituitary-specific gene product called Pit-1 was identified in mice. Pit-1 and its human homologue POUF1 are also homeodomain transcription factors. POUF1 is absolutely required for the differentiation of thyrotropes, somatotropes, and lactotropes, and it directly stimulates the transcription and expression of TSH, GH, and prolactin. Affected individuals with POUF1 mutations have dwarfism and mental retardation. The orphan nuclear hormone receptor-related transcription factor steroidogenic factor-1 (SF-1) was originally identified in the adrenal cortex and gonads as a regulator of steroidogenic enzyme gene expression. However, SF-1 is also expressed in GnRH neurons in the hypothalamus and pituitary gonadotropes. SF-1 regulates the transcription of LH and FSH. Mutations in the SF-1 gene disrupt adrenal and gonadal function, including the loss of gonadotropes in the pituitary gland. Tpit is a transcription factor involved in the differentiation of corticotropes. Tpit interacts with other transcription factors to promote the differentiation of corticotropes and expression of the POMC gene (see later). Mutations in the human Tpit gene result in isolated ACTH deficiency (i.e., other cell types in the body that also express the POMC gene are not affected). This results in a form of secondary adrenal insufficiency that requires life-long replacement with glucocorticoids (see Chapter 42). |
 |
| Before discussing the individual hormones of the adenohypophysis, it is important to understand the
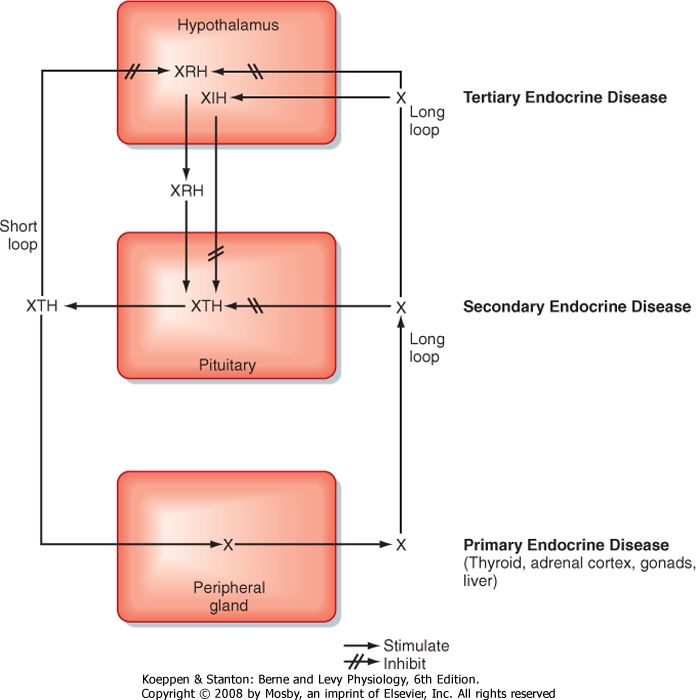
structural and functional organization of the adenohypophysis within the endocrine axes (discussed briefly in Chapter 37; also refer to Table 40-1 and Fig. 40-6). Each endocrine axis is composed of three levels of endocrine cells: (1) hypothalamic neurons, (2) anterior pituitary cells, and (3) peripheral endocrine glands. Hypothalamic neurons release specific hypothalamic releasing hormones (XRHs) that stimulate the secretion of specific pituitary tropic hormones (XTHs). In some cases, production of a pituitary tropic hormone is secondarily regulated by a release-inhibiting hormone (XIH). Pituitary tropic hormones then act on specific peripheral target endocrine glands and stimulate them to release peripheral hormones (X). The peripheral hormone X has two general functions: it regulates several aspects of human physiology, and it negatively feeds back on the pituitary gland and hypothalamus to inhibit the production and secretion of tropic hormones and releasing hormones, respectively (Fig. 40-6).
|
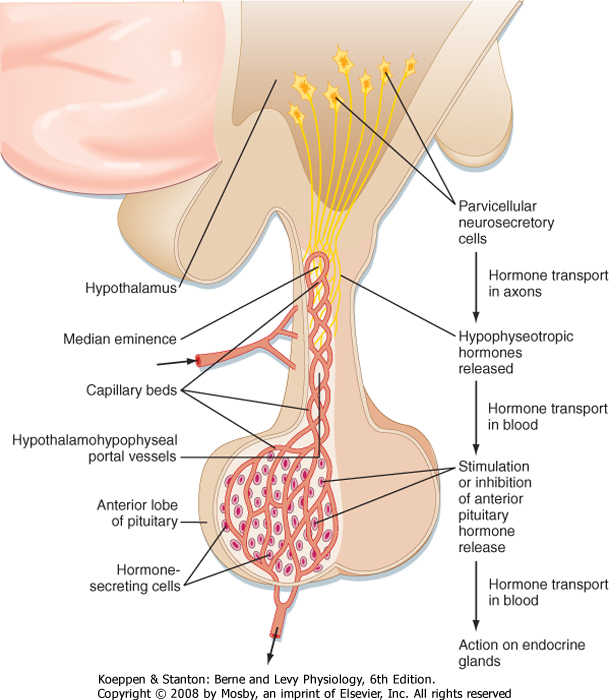
| The hypothalamic level of regulation is neurohormonal. Collections of neuronal cell bodies (called nuclei) reside in several regions of the hypothalamus and are collectively referred to as the hypophysiotropic (i.e., "stimulatory to the hypophysis" [= pituitary]) region of the hypothalamus. These nuclei are distinguished from the magnocellular neurons of the PVN and SON that project to the pars nervosa in that they have small, or parvicellular, neuronal cell bodies that project axons to the median eminence. Parvicellular neurons secrete releasing hormones from their axonal termini at the median eminence (Fig. 40-7). The releasing hormones enter a primary plexus of fenestrated capillaries and are then conveyed to a second capillary plexus located in the pars distalis by the hypothalamohypophysial portal vessels (a "portal" vessel is defined as a vessel that begins and ends in capillaries without going through the heart). At the secondary capillary plexus, the releasing hormones diffuse out of the vasculature and bind to their specific receptors on specific cell types within the pars distalis. The neurovascular link (i.e., the pituitary stalk) between the hypothalamus and pituitary is somewhat fragile and can be disrupted by physical trauma, surgery, or hypothalamic disease. Damage to the stalk and subsequent functional isolation of the anterior pituitary result in a decline in all anterior pituitary tropic hormones except prolactin (see later).
|
| The cells of the adenohypophysis make up the intermediate level of an endocrine axis. The adenohypophysis secretes protein hormones that are referred to as tropic hormones-ACTH, TSH, FSH, LH, GH, and PRL (Table 40-1). With a few exceptions, tropic hormones bind to their cognate receptors on peripheral endocrine glands. Because of this arrangement, pituitary tropic hormones generally do not directly regulate physiological responses (see Chapter 37).
|
|
Table 40-1.
Cell Types of the Adenohypophysis: Hormonal Production and Action, Hypothalamic Regulation, and Feedback Regulation |
| Basophils | Acidophils |
| | Corticotrope | Thyrotrope | Gonadotrope | Somatotrope | Lactotrope |
| Primary hypothalamic regulation | Corticotropin-releasing hormone (CRH): 41-amino acid peptide, stimulatory | Thyrotropin-releasing hormone (TRH): tripeptide, stimulatory | Gonadotropin-releasing hormone (GnRH): decapeptide, stimulatory | Growth hormone-releasing hormone (GHRH): 44-amino acid peptide, stimulatory
Somatostatin: tetradecapeptide, inhibitory | Dopamine (catecholamine):inhibitory
PRL-releasing factor?: stimulatory |
| Tropic hormone secreted | Adrenocorticotropic hormone (ACTH): 4.5-kDa protein | Thyroid-stimulating hormone (TSH): 28-kDa glycoprotein hormone | Follicle-stimulating hormone and luteinizing hormone (FSH, LH): 28- and 33-kDa glycoprotein hormones | Growth hormone (GH): ≈22-kDa protein | Prolactin (PRL): ≈23-kDa protein) |
| Receptor | MC2R (Gs-linked GPCR) | TSH receptor (Gs-linked GPCR) | FSH and LH receptors (Gs-linked GPCRs) | GH receptor (JAK/STAT-linked cytokine receptor) | PRL receptor (JAK/STAT-linked cytokine receptor) |
| Target endocrine gland | Zona fasciculata and zona reticularis of the adrenal cortex | Thyroid epithelium | Ovary (theca and granulosa*)
Testis (Leydig and Sertoli cells) | Liver (but also directactions-especially in terms of metabolic effects) | No endocrine target organ-not part of an endocrine axis |
| Peripheral hormone involved in negative feedback | Cortisol | Triiodothyronine | Estrogen,† progesterone, testosterone, and inhibin‡ | IGF-I GH (short loop) | None |
*Both follicular and luteinized thecal and granulosa cells.
†Estrogen can also have a positive feedback in women.
‡Inhibin selectively inhibits release of FSH from the gonadotrope.
|
| Figure 40-6 Negative-feedback loops regulating hormone secretion in a typical hypothalamus-pituitary-peripheral gland axis. X, peripheral gland hormone; XIH, hypothalamic-inhibiting hormone; XRH, hypothalamic-releasing hormone; XTH, pituitary tropic hormone. |
| page 711 |  | | page 712 |
| page 712 |  | | page 713 |
The endocrine axes have the following important features:
- The activity of a specific axis is normally maintained at a set point, which varies from individual to individual, usually within a normal range. The set point is determined primarily by the integration of hypothalamic stimulation and peripheral hormone negative feedback. Importantly, the negative feedback is not exerted primarily by the physiological responses regulated by a specific endocrine axis, but from the peripheral hormone acting on the pituitary and hypothalamus (Fig. 40-6). Thus, if the level of a peripheral hormone drops, secretion of hypothalamic releasing hormones and pituitary tropic hormones will increase. As the level of peripheral hormone rises, the hypothalamus and pituitary will decrease secretion because of negative feedback. Although certain nonendocrine physiological parameters (e.g., acute hypoglycemia) can regulate some endocrine axes, the axes function semiautonomously with respect to the physiological changes
that they produce. This configuration means that a peripheral hormone (e.g., thyroid hormone) can regulate multiple organ systems without these organ systems exerting competing negative-feedback regulation on the hormone. Clinically, this partial autonomy means that multiple aspects of a patient's physiology are at the mercy of whatever derangements exist within a specific axis.
- Hypothalamic hypophysiotropic neurons are often secreted in a pulsatile manner and are entrained to daily and seasonal rhythms through CNS input. Additionally, hypothalamic nuclei receive a variety of neuronal input from higher and lower levels of the brain. These can be short-term (e.g., various stress/infections) or long-term (e.g., onset of reproductive function at puberty). Thus, inclusion of the hypothalamus in an endocrine axis allows the integration of a considerable amount of information for determining or changing the set point of that axis (or both). Clinically, this means that a broad range of complex neurogenic states can alter pituitary function. Psychosocial dwarfism is a striking example in which children who are abused or under intense emotional stress have lower growth rates as a result of decreased growth hormone secretion by the pituitary gland.
- Abnormally low or high levels of a peripheral hormone (e.g., thyroid hormone) may be due to a defect at the level of the peripheral endocrine gland (e.g., thyroid), the pituitary gland, or the hypothalamus. Such lesions are referred to as primary, secondary, and tertiary endocrine disorders, respectively (Fig. 40-6). A thorough understanding of the feedback relationships within an axis allows the physician to determine where the defect lies. Primary endocrine deficiencies tend to be the most severe because they often involve complete absence of the peripheral hormone.
|
|
| Figure 40-7 Neurovascular link between the hypothalamus and the anterior lobe (pars distalis) of the pituitary. Parvicellular "hypophysiotropic" neurosecretory neurons within various hypothalamic nuclei project axons to the median eminence, where they secrete releasing hormones (RHs). RHs flow down the pituitary stalk in the hypothalamohypophyseal portal vessels to the anterior pituitary. RHs (and release-inhibiting hormones-see text) regulate the secretion of tropic hormones from the five cell types of the anterior pituitary. (From Larsen PR et al [eds]: Williams Textbook of Endocrinology, 10th ed. Philadelphia, Saunders, 2003.) |
| page 713 |  | | page 714 |
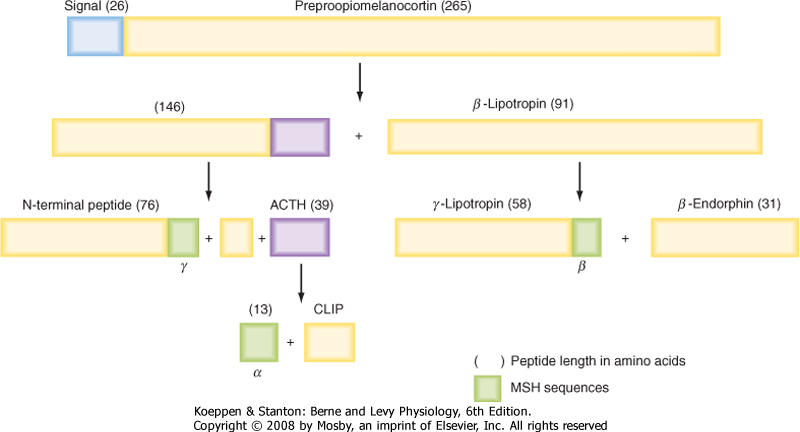
| Figure 40-8 The original gene transcript of proopiomelanocortin contains structures of multiple bioactive compounds. ACTH, adrenocorticotropic hormone; CLIP, corticotropin-like intermediate peptide; MSH, melanocyte-stimulating hormone. Note that ACTH is the only bioactive peptide released by the human corticotrope. |
| Endocrine Function of the Adenohypophysis
|
| The adenohypophysis consists of the following endocrine cell types: corticotropes, thyrotropes, gonadotropes, somatotropes, and lactotropes (Table 40-1).
|
| Corticotropes stimulate (i.e., are "tropic to") the adrenal cortex as part of the hypothalamic-pituitary-adrenal (HPA) axis. Corticotropes produce the hormone adrenocorticotropic hormone (ACTH; also called corticotropin), which stimulates two zones of the adrenal cortex (see Chapter 42). ACTH is a 39-amino acid peptide that is synthesized as part of a larger prohormone, proopiomelanocortin (POMC). Thus, corticotropes are also referred to as POMC cells. POMC harbors the peptide sequence for ACTH, forms of melanocyte-stimulating hormone (MSH), endorphins (endogenous opioids), and enkephalins (Fig. 40-8). The human corticotrope expresses only the prohormone convertase, which produces ACTH as the sole active hormone secreted from these cells. The other fragments that are cleaved from POMC are the N-terminal fragment and β-lipotropic hormone (β-LPH). Neither of these fragments play a physiological role in humans.
|
| ACTH circulates as an unbound hormone and has a short half-life of about 10 minutes. It binds to the melanocortin 2 receptor (MC2R) on cells in the adrenal cortex (Fig. 40-9). ACTH acutely increases cortisol and adrenal androgen production, increases the expression of steroidogenic enzyme genes, and in the long term, promotes the growth and survival of two zones in the adrenal cortex (see Chapter 42).
|
| At supraphysiological levels, ACTH causes darkening of light-colored skin (e.g., Cushing's disease). Normally, keratinocytes express the POMC gene but process it to α-MSH instead of ACTH. Keratinocytes secrete α-MSH in response to ultraviolet light, and α-MSH acts as a paracrine factor on neighboring melanocytes to darken the skin. α-MSH binds to the MC1R on melanocytes. However, at high levels, ACTH can also cross-react with the MC1R receptor on skin melanocytes (Fig. 40-9). Thus, darkening of skin is one indicator of excessive ACTH levels. |
| ACTH is under stimulatory control by the hypothalamus. A subset of parvicellular hypothalamic neurons expresses the peptide procorticotropin-releasing hormone (pro-CRH) (Table 40-1). Pro-CRH is processed to an amidated 41-amino acid peptide, CRH. CRH acutely stimulates ACTH secretion and increases transcription of the POMC gene. The parvicellular neurons that express CRH also coexpress ADH, and ADH potentiates the action of CRH on corticotropes.
|
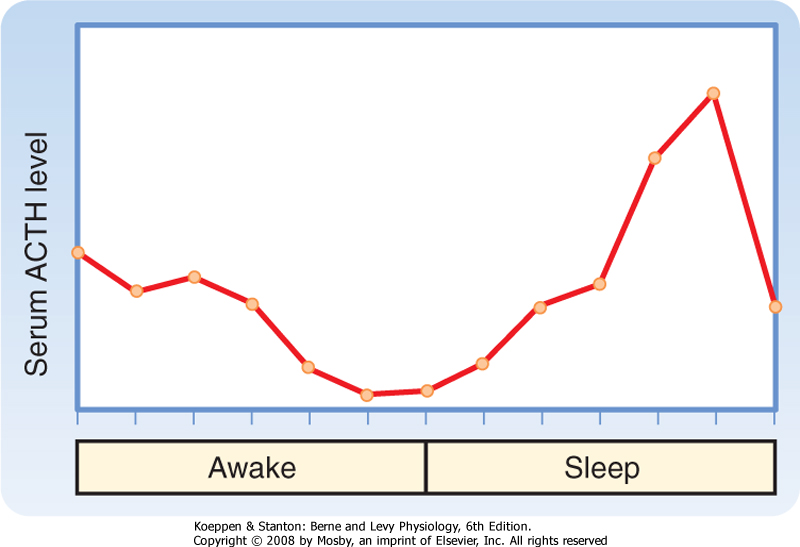
| ACTH secretion has a pronounced diurnal pattern, with a peak in early morning and a valley in late afternoon (Fig. 40-10). In addition, secretion of CRH-and hence secretion of ACTH-is pulsatile.
|
| page 714 |  | | page 715 |
| Figure 40-9 Normal levels of ACTH act on the MC2R to increase cortisol. Supraphysiological levels of ACTH act on both the MC2R and the MC1R on melanocytes and cause skin darkening. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| Figure 40-10 Diurnal pattern of serum ACTH. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| page 715 |  | | page 716 |
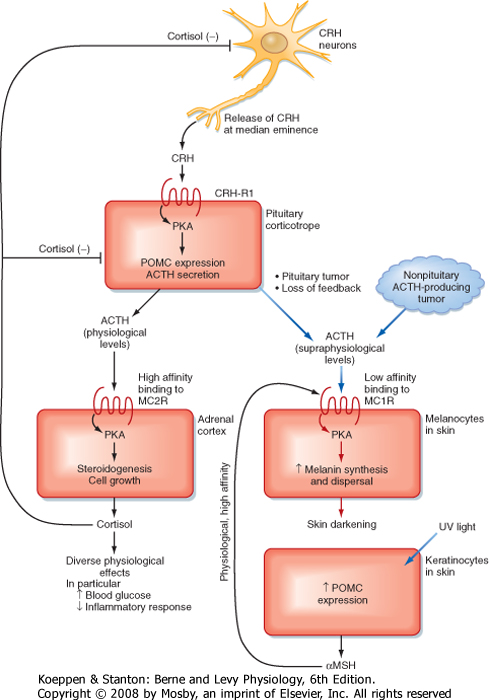
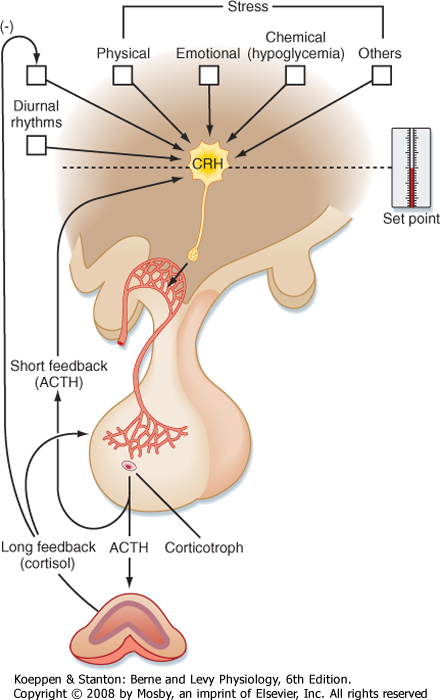
| Figure 40-11 Hypothalamus-pituitary-adrenal axis illustrating factors regulating the secretion of corticotropin-releasing hormone (CRH). ACTH, adrenocorticotropic hormone. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| There are multiple regulators of the HPA axis, and many of them are mediated through the CNS (Fig. 40-11). Many types of stress, both neurogenic (e.g., fear) and systemic (e.g., infection), stimulate secretion
of ACTH. The stress effects are mediated through CRH and ADH and the CNS. The response to many forms of severe stress can persist despite negative feedback from high cortisol levels. This means that the hypothalamus has the ability to reset the "set point" of the HPA axis in response to stress. Severe, chronic depression can reset the HPA axis as a result of hypersecretion
of CRH and is a factor in the development of tertiary hypercortisolism. Cortisol exerts negative feedback on the pituitary, where it suppresses POMC gene expression and ACTH secretion, and on the hypothalamus, where it decreases pro-CRH gene expression and release of CRH. Because cortisol has profound effects on the immune system (see Chapter 42), the HPA axis and the immune system are closely coupled. Moreover, cytokines, particularly interleukin-1 (IL-1), IL-2, and IL-6, stimulate the HPA axis.
|
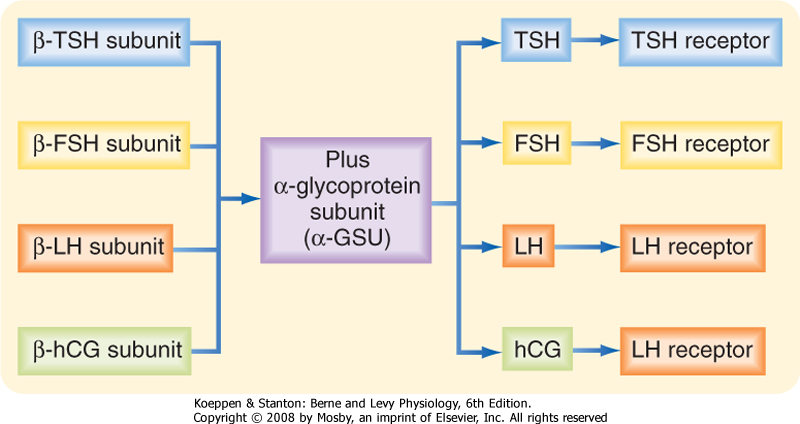
| Thyrotropes regulate thyroid function by secreting the hormone thyroid-stimulating hormone (TSH; also called thyrotropin) as part of the hypothalamus-pituitary-thyroid axis. TSH is one of three pituitary glycoprotein hormones (Table 40-1), which also include follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (see later). TSH is a heterodimer composed of an α subunit, called the α-glycoprotein subunit (α-GSU), and a β subunit (β-TSH) (Fig. 40-12). The α-GSU is common to TSH, FSH, and LH, whereas the β subunit is specific to the hormone (i.e., β-TSH, β-FSH, and β-LH are all unique). Glycosylation of the subunits increases their stability in circulation and enhances the affinity and specificity of the hormones for their receptors. The half-lives of TSH, FSH, and LH (and an LH-like placental glycoprotein hormone, human chorionic gonadotropin [hCG]) are relatively long, ranging from tens of minutes to several hours.
|
| TSH binds to the TSH receptor on thyroid epithelial cells (see Chapter 41). As discussed in Chapter 41, the production of thyroid hormones is a complex, multistep process. TSH stimulates essentially every aspect of thyroid function. TSH also has a strong tropic effect and stimulates hypertrophy, hyperplasia, and survival of thyroid epithelial cells. In geographical regions where the availability of iodide is limited (iodide is required for the synthesis of thyroid hormone), TSH levels are elevated because of reduced negative feedback. Elevated TSH levels can produce noticeable growth of the thyroid and a bulge in the neck called a goiter.
|
| Figure 40-12 Pituitary glycoprotein hormones. hCG is made by the placenta (see Chapter 43) and binds to the LH receptor. FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin; LH, luteinizing hormone; TSH, thyroid-stimulating hormone. |
| page 716 |  | | page 717 |
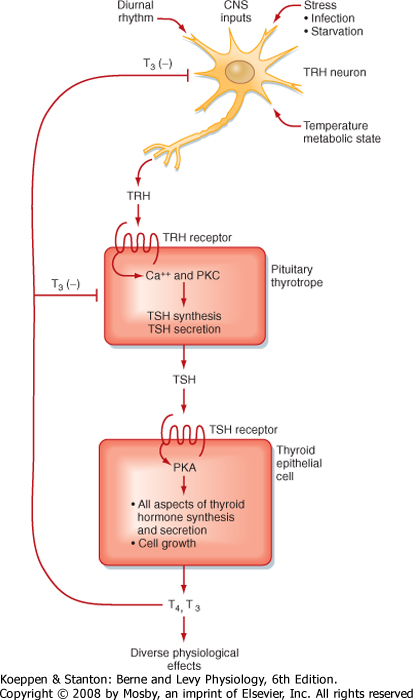
| Figure 40-13 Hypothalamus-pituitary-thyroid axis. PKA, protein kinase A; PKC, protein kinase C; T3, triiodothyronine (active form of thyroid hormone); T4, tetraiodothyronine; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| During embryonic development, GnRH neurons migrate to the mediobasal hypothalamus from the nasal placode. Patients with Kallmann's syndrome have tertiary hypogonadotropic hypogonadism, often associated with loss of the sense of smell (anosmia). This is due to a mutation in the KAL gene, which results in failure of the GnRH neuronal precursors to properly migrate to the hypothalamus and establish a neurovascular link to the pars distalis. |
 |
| The pituitary thyrotrope is stimulated by the releasing hormone thyrotropin-releasing hormone (TRH) (Table 40-1). TRH, produced by a subset of parvicellular hypothalamic neurons, is a tripeptide with cyclization of a glutamine at its N-terminus (pyro-Glu) and an amidated C-terminus. TRH is synthesized as a larger
prohormone that contains six copies of TRH within its sequence. It binds to the TRH receptor on thyrotropes (Fig. 40-13). TRH neurons are regulated by numerous CNS-mediated stimuli, and TRH is released according to a diurnal rhythm (highest during overnight hours, lowest around dinnertime). TRH is regulated by various types of stress, but unlike CRH, stress inhibits secretion of TRH. Such stress includes physical stress, starvation, and infection. The active form of thyroid hormone, triiodothyronine (T3), negatively feeds back on both pituitary thyrotropes and TRH-producing
neurons. T3 represses both β-TSH expression and the sensitivity of thyrotropes to TRH. T3 also inhibits TRH production and secretion.
|
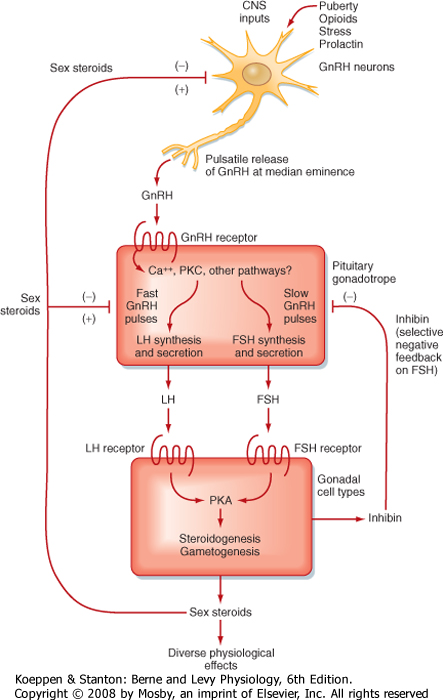
| The gonadotrope secretes FSH and LH (also called gonadotropins) and regulates the function of gonads in both sexes. As such, the gonadotrope plays an integral role in the hypothalamus-pituitary-testis axis and the hypothalamus-pituitary-ovary axis (Fig. 40-14).
|
| FSH and LH are segregated into different secretory granules and are not cosecreted in equimolar amounts (in contrast to ADH and neurophysin, for example). This allows independent secretion of FSH/LH by gonadotropes. The actions of FSH and LH on gonadal function are complex, especially in women, and will be discussed in detail in Chapter 43. In general, gonadotropins promote testosterone secretion in men and estrogen and progesterone secretion in women. FSH also increases the secretion of a transforming growth factor-β (TGF-β)-related protein hormone called inhibin in both sexes.
|
| FSH and LH secretion are regulated by one hypothalamic releasing hormone, gonadotropin-releasing hormone (GnRH; also called LHRH). GnRH is a 10-amino acid peptide produced by a subset of parvicellular hypothalamic GnRH neurons (Fig. 40-14). GnRH is produced as a larger prohormone and, as part of its processing to a decapeptide, is modified with a cyclized glutamine (pyro-Glu) at its amino-terminus and an amidated carboxy-terminus.
|
| page 717 |  | | page 718 |
| Figure 40-14 Hypothalamus-pituitary-gonadal axis. FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone. (Modified from Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
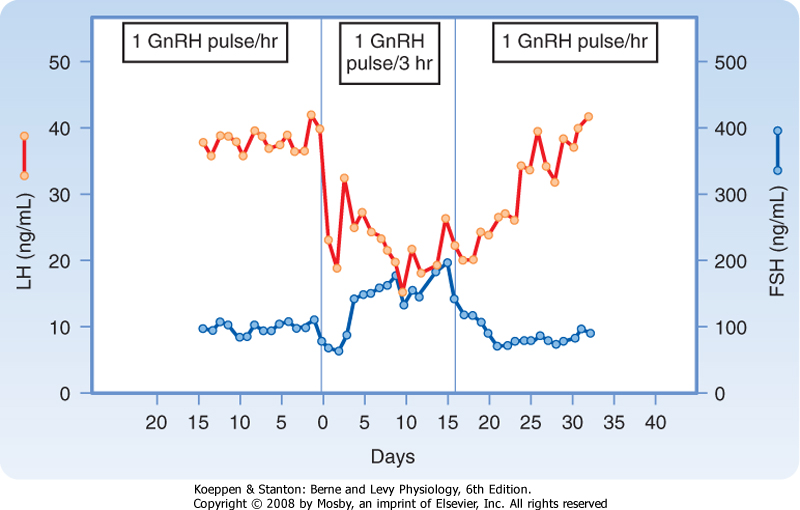
| GnRH is released in a pulsatile manner (Fig. 40-15), and both the pulsatile secretion and the frequency of the pulses have important effects on the gonadotrope. Continuous infusion of GnRH down-regulates the GnRH receptor, thereby resulting in a decrease in FSH and LH secretion. In contrast, pulsatile secretion does not desensitize the gonadotrope to GnRH, and FSH and LH secretion is normal. At a frequency of one pulse per hour, GnRH preferentially increases LH secretion (Fig. 40-16). At a slower frequency of one pulse per 3 hours, GnRH preferentially increases FSH secretion. Gonadotropins increase sex steroid synthesis (Fig. 40-14). In men, testosterone and estrogen negatively feed back at the level of the pituitary and the hypothalamus. Exogenous progesterone also inhibits gonadotropin function in men and is being considered as a possible ingredient in a male contraceptive pill. Additionally,
inhibin negatively feeds back selectively on FSH secretion in men and women. In women, progesterone and testosterone negatively feed back on gonadotropic function at the level of the hypothalamus and pituitary. At low doses, estrogen also exerts negative feedback on FSH and LH secretion. However, high estrogen levels maintained for 3 days cause a surge in LH and, to a lesser extent, FSH secretion. This positive feedback is observed at the hypothalamus and pituitary. At the hypothalamus, GnRH pulse amplitude and frequency increase. At the pituitary, high estrogen levels greatly increase the sensitivity of the gonadotrope to GnRH, both by increasing GnRH receptor levels and by enhancing postreceptor signaling pathway components (see Chapter 43).
|
| page 718 |  | | page 719 |
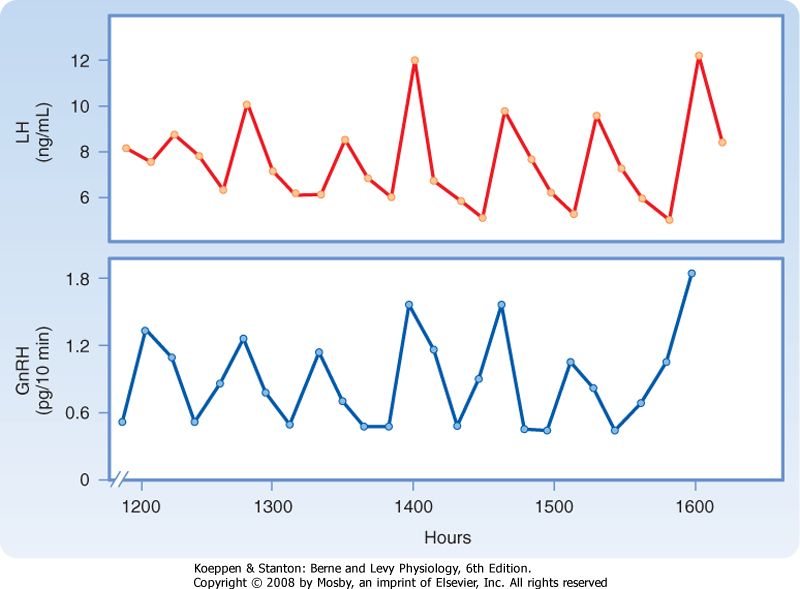
| Figure 40-15 Fluctuation of peripheral vein plasma LH levels and portal vein plasma GnRH levels in unanesthetized, ovariectomized female sheep. Each pulse of LH is coordinated with a pulse of GnRH. This supports the view that pulsatility of LH release is dependent on pulsatile stimulation of the pituitary by GnRH. (From Levine J et al: Endocrinology 111:1449, 1982.) |
| Figure 40-16 Frequency-encoded regulation of FSH and LH secretion from gonadotropes. A high frequency of GnRH (1 pulse/hr) preferentially stimulates LH secretion, whereas a slower frequency of GnRH promotes FSH secretion. (From Larsen PR et al [eds]: Williams Textbook of Endocrinology, 10th ed. Philadelphia, Saunders, 2003.) |
| The somatotrope produces growth hormone (GH, also called somatotropin) and is part of the hypothalamus-pituitary-liver axis (Fig. 40-17). A major target of GH is the liver, where it stimulates the production of insulin-like growth factor type I (IGF-I). GH is a 191-amino acid protein that is similar to prolactin (PRL) and human placental lactogen (hPL); accordingly, there is some overlap in activity among these hormones. Multiple forms of GH are present in serum and constitute a "family of hormones," with the 191-amino acid (22-kDa) form representing approximately 75% of the circulating GH. The GH receptor is a member of the cytokine/GH/PRL/erythropoietin receptor family and, as such, is linked to the JAK/STAT signaling
pathway (see Chapter 3). Human GH can also act as an agonist for the PRL receptor. About 50% of the 22-kDa form of GH in serum is bound to the N-terminal portion (the extracellular domain) of the GH receptor and is called GH-binding protein (GHBP). Laron dwarfs, who lack normal GH receptors but have normal GH secretion, do not have detectable GHBP in their serum. GHBP reduces renal clearance and thus increases the biological half-life of GH, which is about 20 minutes. The liver and kidney are major sites of GH degradation.
|
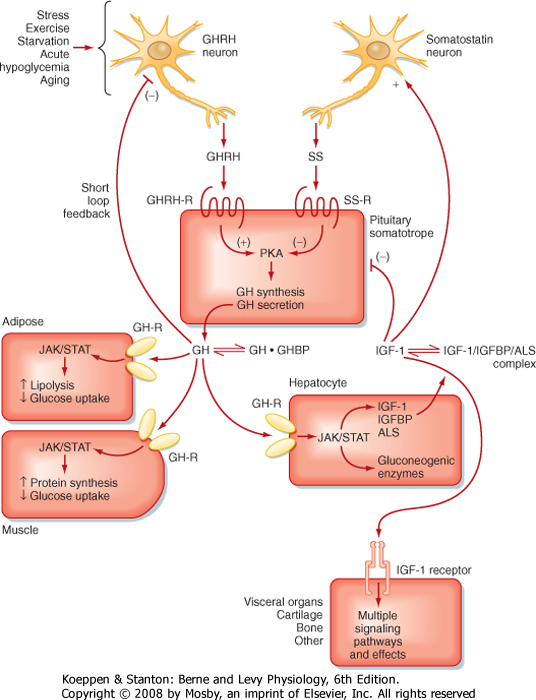
| GH secretion is under dual control by the hypothalamus (Fig. 40-17). The hypothalamus predominantly stimulates GH secretion via the peptide growth hormone-releasing hormone (GHRH). This hormone is a member of the vasoactive intestinal polypeptide (VIP)/secretin/glucagon family and is processed into a 44-amino acid peptide with an amidated C-terminus from a larger prohormone. GHRH enhances GH secretion and GH gene expression. The hypothalamus inhibits pituitary GH synthesis and release via the peptide somatostatin. Somatostatin, in the anterior pituitary, inhibits the release of GH and TSH. GH secretion is also regulated by ghrelin, which is primarily produced by the stomach but is also expressed in the hypothalamus. Ghrelin increases appetite and may serve as a signal to coordinate nutrient acquisition with growth.
|
| The primary negative feedback on the somatotrope is exerted by IGF-I (Fig. 40-17). GH stimulates IGF-I production by the liver, and IGF-I then inhibits GH synthesis and secretion by the pituitary and hypothalamus in a classic "long" feedback loop. In addition, GH itself exerts negative feedback on release of GHRH through a "short" feedback loop. GH also increases somatostatin release.
|
| page 719 |  | | page 720 |
| Figure 40-17 Hypothalamus-pituitary-liver axis. ALS, acid labile subunit; GHBP, growth hormone-binding protein; GHRH, growth hormone-releasing hormone; IGFBP, insulin-like growth factor-binding protein; IGF-I, insulin-like growth factor I; SS, somatostatin. (From Porterfield SP, White BA: Endocrine Physiology, 3rd ed. Philadelphia, Mosby, 2007.) |
| GH secretion, like that of ACTH, shows prominent diurnal rhythms, with peak secretion occurring in the early morning just before awakening. Its secretion is stimulated during deep, slow-wave sleep (stages III and IV). GH secretion is lowest during the day. This rhythm is entrained to sleep-wake patterns rather than light-dark patterns, so a phase shift occurs in people who work night shifts. As is typical of anterior pituitary hormones, GH secretion is pulsatile. Levels of GH in serum vary widely (0 to 30 ng/mL, with most values usually falling between 0 and 3). Because of this marked variation, serum GH values are of minimal clinical value unless the sampling time is known. Frequently, rather than measuring GH, the clinician measures IGF-I because its secretion is regulated by GH and IGF-I has a relatively long circulating half-life that minimizes pulsatile and diurnal changes in secretion.
|
| page 720 |  | | page 721 |
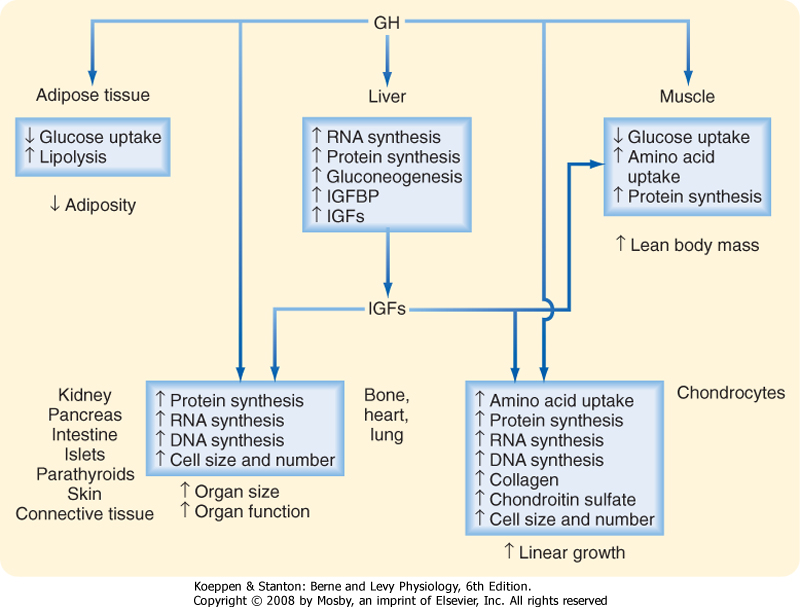
| Figure 40-18 Biological actions of GH. The effects on linear growth, organ size, and lean body mass are at least partly mediated by insulin-like growth factors (IGFs) (somatomedins) produced in the liver and in the GH target tissues as well. IGFBP, insulin-like growth factor-binding protein. |
| GH secretion is also regulated by several different physiological states. GH is classified as one of the "stress" hormones and is increased by neurogenic and physical stress. It promotes lipolysis, increases protein synthesis, and antagonizes the ability of insulin to reduce blood glucose levels. It is not surprising, therefore, that acute hypoglycemia is a stimulus for GH secretion, and GH is classified as a hyperglycemic hormone. A rise in the serum concentration of some amino acids also stimulates GH secretion. In contrast, an increase in blood glucose or free fatty acids inhibits secretion of GH. Obesity also inhibits GH secretion, in
part because of insulin resistance (relative hyperglycemia) and increased circulating free fatty acids. Conversely, exercise and starvation stimulate GH secretion. Other hormones that regulate GH secretion include estrogen, androgens, and thyroid hormone, which enhance GH and IGF-I secretion, as well as bone maturation.
|
| Direct versus Indirect Actions of Growth Hormone
|
| GH acts directly on the liver, muscle, and adipose tissue to regulate energy metabolism (Fig. 40-18). It shifts metabolism to lipid use for energy, thereby conserving carbohydrates and proteins. GH is a protein anabolic hormone that increases cellular amino acid uptake and incorporation into protein, and it represses proteolysis. Consequently, it produces nitrogen retention (positive nitrogen balance) and decreases urea production. The muscle wasting that occurs concomitant with aging has been proposed to be caused, at least in part, by the decrease in GH secretion that occurs with aging.
|
| GH is a lipolytic hormone. It activates hormone-sensitive lipase and therefore mobilizes neutral fats from adipose tissue. As a result, serum fatty acid levels rise after GH administration, more fats are used for energy production, and fatty acid uptake and oxidation increase in skeletal muscle and liver. GH can be ketogenic as a result of the increase in fatty acid oxidation (the ketogenic effect of GH is not seen when insulin levels are normal). If insulin is given along with GH, the lipolytic effects of GH are abolished.
|
| GH alters carbohydrate metabolism. Many of its actions may be secondary to increased fat mobilization and oxidation. (Remember, an increase in serum free fatty acids inhibits uptake of glucose in skeletal muscle and adipose tissue.) After administration of GH, blood glucose rises. The hyperglycemic effects of GH are mild and slower than those of glucagon and epinephrine. The increase in blood glucose results in part from decreased glucose uptake and use in skeletal muscle and adipose tissue. Liver glucose output increases, and this is probably not a result of glycogenolysis. In fact, glycogen levels can rise after administration of GH. However, the increase in fatty acid oxidation and hence the rise in liver acetyl coenzyme A (acetyl CoA) stimulate gluconeogenesis, followed by increased glucose production from substrates such as lactate and glycerol.
|
| GH antagonizes the action of insulin at the postreceptor level in skeletal muscle and adipose tissue (but not the liver). Hypophysectomy (removal of the pituitary gland) can improve diabetic management because GH, like cortisol, decreases insulin sensitivity. Because GH produces insulin insensitivity, it is considered a diabetogenic hormone. When secreted in excess, GH can cause diabetes mellitus, and the insulin levels necessary to maintain normal metabolism increase. Excessive insulin secretion resulting from an excess of GH can cause damage to pancreatic beta cells. In the absence of GH, insulin secretion declines. Thus, normal levels of GH are required for normal pancreatic function and insulin secretion.
|
| Indirect Effects of Growth Hormone on Growth
|
| GH increases skeletal and visceral growth; children without GH show growth stunting or dwarfism. GH also promotes cartilage growth, long-bone length, and periosteal growth. Most of these effects are mediated by a group of hormones called insulin-like growth factors.
|
| page 721 |  | | page 722 |
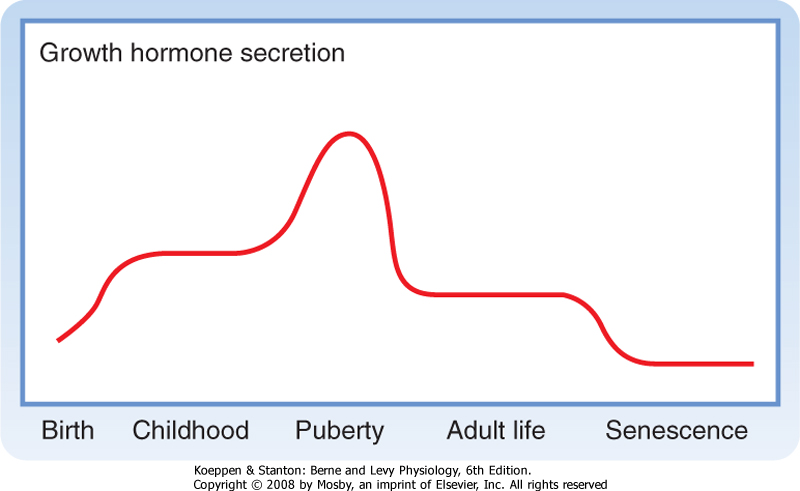
| Figure 40-19 Lifetime pattern of GH secretion. GH levels are higher in children than in adults, with a peak period during puberty. GH secretion declines with aging. |
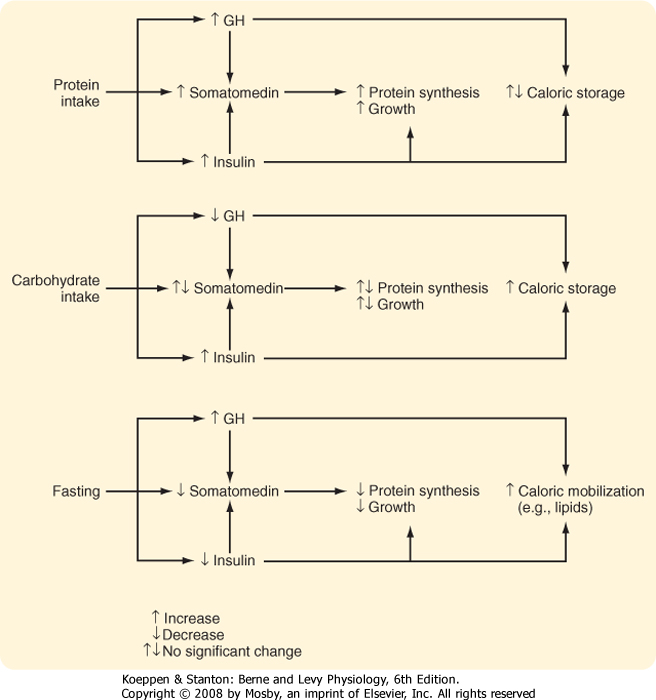
| When ample supplies of nutrients are available, high serum amino acid levels stimulate GH and insulin secretion, and high serum glucose levels stimulate insulin secretion. The high serum GH, insulin, and nutrient supply stimulate IGF production, and these conditions are appropriate for growth. However, if the diet is high in calories but low in amino acids, the hormonal response is different. Whereas high carbohydrate availability results in high insulin availability, low serum amino acid levels inhibit GH and IGF production. These conditions allow dietary carbohydrates and fats to be stored, but conditions are unfavorable for tissue growth. On the other hand, during fasting, when the availability of nutrients decreases, serum GH levels rise and serum insulin levels fall (because of hypoglycemia). IGF production is low, and the conditions are not favorable for growth. In these circumstances, the rise in GH secretion is beneficial because it promotes fat mobilization while minimizing tissue protein loss. In the absence of insulin, peripheral tissue glucose use decreases, thereby conserving glucose for essential tissues such as the brain (Fig. 40-20). |
 |
| GH is necessary for growth before adulthood. Deficiencies can produce dwarfism, and excesses can produce gigantism. Normal growth requires not only normal levels of GH but also normal levels of thyroid hormones, insulin, and sex steroids. |
| Dwarfism. If GH deficiency occurs before puberty, growth is severely impaired. Individuals with this condition are relatively well proportioned and have normal intelligence. If the anterior pituitary deficiency is limited to GH, they can have a normal life span. They are sometimes "pudgy" because they lose GH-induced lipolysis. If they have panhypopituitary dwarfism (all anterior pituitary hormones are deficient), with a deficiency of gonadotropins, they may not mature sexually and remain infertile. People with dwarfism show few metabolic abnormalities other than a tendency toward hypoglycemia, insulinopenia, and increased insulin sensitivity. There are multiple potential sites of impairment. GH secretion may be reduced, GH-stimulated IGF production may decrease, or IGF action may be deficient. Laron dwarfs are resistant to GH because of a genetic defect in expression of the GH receptor such that the response to GH is impaired. Thus, although serum GH levels are normal to high, Laron dwarfs do not produce IGFs in response to GH. Treating patients afflicted by Laron dwarfism with GH will not correct the growth deficiency. The African pygmy represents another example of abnormal growth. Individuals with this condition have normal serum GH levels, but they do not exhibit the normal rise in IGF that occurs at puberty. They also may have a partial defect in GH receptors because IGF-I levels do not rise normally after GH is administered. However, IGF-II levels are normal. Unlike Laron dwarfs, they do not totally lack the IGF response to GH. |
| GH deficiency in adults is becoming recognized as a pathological syndrome. If the GH deficiency occurs after the epiphyses close, growth is not impaired. GH deficiency is one of many possible causes of hypoglycemia. Recent studies have shown that extended deficiencies of GH lead to changes in body composition. The percentage of body weight that is fat increases, whereas the percentage that is protein decreases. In addition, muscle weakness and early exhaustion are symptoms of GH deficiency. Because the muscle loss that occurs with aging may result from an age-related decline in GH production (Fig. 40-19), GH is being used experimentally in elderly people to delay the physical decline associated with aging. The efficacy of this treatment in humans has not been established. |
| page 722 |  | | page 723 |
| Figure 40-20 Complementary regulation of GH and insulin secretion coordinates availability of nutrients with anabolism and either caloric storage or mobilization. Note that both hormones are increased by protein and that both stimulate protein synthesis. |
| The IGFs are multifunctional hormones that regulate cellular proliferation, differentiation, and metabolism. These protein hormones resemble insulin in structure and function. The two hormones in this family, IGF-I and IGF-II, are produced in many tissues and have autocrine, paracrine, and endocrine actions. IGF-I is the major form produced in most adult tissues, and IGF-II is the major form produced in the fetus. Both hormones are structurally similar to proinsulin, with IGF-I having 42% structural homology with proinsulin. IGFs and insulin cross-react with each other's receptors, and IGFs in high concentration mimic the metabolic actions of insulin. Both IGF-I and IGF-II act through type I IGF receptors, which are similar to insulin and EGF receptors and contain intrinsic tyrosine kinase. However, IGF-II also binds to the type II IGF/mannose-6-phosphate
receptor. This receptor does not resemble the insulin receptor and does not have intrinsic tyrosine kinase activity. Binding to these receptors probably facilitates internalization and degradation of IGF. IGFs stimulate glucose and amino acid uptake and protein and DNA synthesis. They were initially called somatomedins because they mediate GH (somatotropin)
action on cartilage and bone growth. IGFs have many other actions, and GH is not the only regulator of IGF formation. Initially, IGFs were thought to be produced in the liver in response to a GH stimulus. It is now known that IGFs are produced in many tissues, and many actions are autocrine or paracrine. The liver is probably the predominant source of circulating IGFs (Fig. 40-18).
|
| Essentially all circulating IGFs are transported in serum bound to insulin-like growth factor-binding proteins (IGFBPs). IGFBPs bind to IGFs and then associate with another protein called acid labile subunit (ALS). GH stimulates the hepatic production of IGF-I, IGFBPs, and ALS. The IGFBP/ALS/IGF-I complex mediates transport and bioavailability of IGF-I. Although IGFBPs generally inhibit IGF action, they greatly increase the biological half-life of IGFs (up to 12 hours). IGFBP proteases degrade IGFBP and play a role in locally generating free (i.e., active) IGFs. This is of interest in the context of IGF-responsive cancers (e.g., prostate cancer), which may overexpress one or more IGFBP proteases.
|
| Although GH is an effective stimulator of IGF production, the correlation between GH and IGF-I is greater than the correlation between GH and IGF-II. During puberty, when GH levels increase (Fig. 40-19), IGF-I levels increase in parallel. Insulin also stimulates IGF production, and GH cannot stimulate IGF production in the absence of insulin. Starvation effectively inhibits IGF secretion, even when GH levels are high. PRL or hPL can increase IGF-II secretion in the fetus, and IGF-II is considered a fetal growth regulator. Although GH is a primary stimulus for IGF production in the liver, parathyroid hormone (PTH) and estradiol are more effective stimuli for osteoblastic IGF-I production.
|
| IGFs are mitogenic and have profound effects on bone and cartilage. They stimulate the growth of bones, cartilage, and soft tissue and regulate all aspects of the metabolism of chondrocytes, which are the cartilage-forming cells. Although appositional growth of long bones continues after closure of the epiphyses, growth in length ceases. IGFs stimulate osteoblast replication and the synthesis of collagen and bone matrix. Serum IGF levels correlate well with growth in children.
|
| page 723 |  | | page 724 |
| The lactotrope produces the hormone prolactin, which is a 199-amino acid, single-chain protein. PRL is structurally related to GH and hPL (see Chapter 43).
Like GH, the PRL receptor is a member of the cytokine family coupled to the JAK/STAT signaling pathways. Because the primary action of PRL in humans is related to breast development and function during pregnancy and lactation, the regulation and actions of prolactin will be discussed in detail in Chapter 43.
|
In the context of the pituitary gland, it should be appreciated that the lactotrope differs from the other endocrine cell types of the adenohypophysis in two major ways:
- The lactotrope is not part of an endocrine axis. This means that PRL acts directly on nonendocrine cells (primarily of the breast) to induce physiological changes.
- Production and secretion of PRL are predominantly under inhibitory control by the hypothalamus. Thus, disruption of the pituitary stalk and the hypothalamohypophysial portal vessels (e.g., secondary to surgery or physical trauma) results in an increase in PRL levels but a decrease in ACTH, TSH, FSH, LH, and GH.
|
| PRL circulates unbound to serum proteins and thus has a relatively short half-life of about 20 minutes. Normal basal serum concentrations are similar in men and women. Release of PRL is normally under tonic inhibition by the hypothalamus. This is exerted by dopaminergic tracts that secrete dopamine in the median eminence. There is also evidence for the existence of a prolactin-releasing factor (PRF). The exact nature of this compound is not known, although many factors, including TRH and hormones in the glucagon family (secretin, glucagon, VIP, and gastric inhibitory polypeptide [GIP]) can stimulate the release of PRL.
|
| PRL is one of the many hormones released in response to stress. Surgery, fear, stimuli causing arousal, and exercise are all effective stimuli. As is the case with GH, sleep increases PRL secretion, and PRL has a pronounced sleep-associated diurnal rhythm. However, unlike GH, the rise in sleep-associated PRL is not associated with a specific sleep phase. Drugs that interfere with the synthesis or action of dopamine increase PRL secretion. Many commonly prescribed antihypertensive drugs and tricyclic antidepressants are dopamine inhibitors. Bromocriptine is a dopamine agonist that can be used to inhibit PRL secretion. Somatostatin, TSH, and GH also inhibit PRL secretion.
|
- The pituitary gland (also called the hypophysis) is composed of epithelial tissue (the adenohypophysis or anterior lobe) and neural tissue (the neurohypophysis or posterior lobe).
- Magnocellular hypothalamic neurons in the paraventricular and supraoptic nuclei project axons down the infundibular stalk and terminate in the pars nervosa. The pars nervosa is a neurovascular organ in which neurohormones are released and diffuse into the vasculature.
- Two neurohormones, ADH and oxytocin, are synthesized in the hypothalamus in the magnocellular neuronal cell bodies. ADH and oxytocin are transported intraaxonally down the hypothalamohypophyseal tracts to the pars nervosa. Stimuli perceived by the cell bodies and dendrites in the hypothalamus control the release of ADH and oxytocin at the pars nervosa.
- The adenohypophysis secretes several tropic hormones that are part of the endocrine axes. An endocrine axis includes the hypothalamus, the pituitary, and a peripheral endocrine gland. The set point of an axis is largely controlled by negative feedback of the peripheral hormone on the pituitary and hypothalamus.
- The adenohypophysis contains five endocrine cell types: corticotropes, thyrotropes, gonadotropes, somatotropes, and lactotropes. Corticotropes secrete ACTH, thyrotropes secrete TSH, gonadotropes secrete FSH and LH, somatotropes secrete GH, and lactotropes secrete PRL.
- The hypothalamus regulates the anterior pituitary by secreting releasing hormones. These small peptides are carried via the hypophyseal portal system to the anterior pituitary, where they control synthesis and release of the pituitary hormones ACTH, TSH, FSH, LH, and GH. PRL secretion is inhibited by the hypothalamus through the catecholamine dopamine.
- GH stimulates growth primarily through regulation of the growth-promoting hormones IGF-I and IGF-II. GH raises blood glucose levels by decreasing peripheral tissue utilization and is protein anabolic and lipolytic.
- PRL initiates and maintains lactation.
|
 |
|