| 29 The Small Intestinal Phase of the Integrated Response to a Meal
|
| The small intestine is the critical portion of the intestinal tract for assimilation of nutrients. In this site the meal is mixed with a variety of secretions that permit its digestion and absorption, and motility functions serve to ensure adequate mixing and exposure of the intestinal contents (chyme) to the absorptive surface. The small intestine has many specializations that enable it to perform its functions efficiently. One of the most obvious specializations is the substantial surface area of the mucosa. This is achieved in a number of different ways: the small intestine is essentially a long tube that is coiled inside the abdominal cavity, there are folds of the full thickness of the mucosa and submucosa, the mucosa has finger-like projections called villi, and finally, each epithelial cell has microvilli on its apical surface. Thus, a large surface area exists over which digestion and absorption occur.
|
| The main characteristic of the small intestinal phase of the response to a meal is controlled delivery of chyme from the stomach to match the digestive and absorptive capacity of the intestine. In addition, there is further stimulation of pancreatic and biliary secretion and emptying of these secretions into the small intestine. Therefore, the function of this region is highly regulated by feedback mechanisms that involve hormonal, paracrine, and neural pathways.
|
| The stimuli that regulate these processes are both mechanical and chemical and include distention of the intestinal wall and the presence of protons, high osmolarity, and nutrients in the intestinal lumen. These stimuli result in a set of changes that represent the intestinal phase of the response to the meal: (1) increased pancreatic secretion, (2) increased gallbladder contraction, (3) relaxation of the sphincter of Oddi, (4) regulation of gastric emptying, (5) inhibition of gastric acid secretion, and (6) interruption of the migrating motor complex (MMC). The goal of this chapter is to discuss how such changes are brought about and how they result ultimately in the assimilation of nutrients. Changes in small intestinal function that occur after the meal has passed through will also be touched on.
|
| GASTRIC EMPTYING IN THE SMALL INTESTINAL PHASE
|
| The gastrointestinal (GI) tract plays a major role in the sensing and signaling of ingested nutrients by activating neural and endocrine pathways that connect with other signals, such as fat energy storage and utilization, which together regulate energy homeostasis. Satiety signals from the GI tract are generally involved in the short-term regulation of food intake, such as individual meal size and meal duration. For example, the luminal contents activate vagal afferent pathways leading to suppression of meal size. In addition, several GI hormones released by nutrients also influence food intake. Cholecystokinin (CCK) is a well-described satiety hormone; it is released by nutrients and decreases food intake after exogenous administration. Other GI hormones in this class include glucagon-like peptide 1 (GLP-1) and peptide YY (PYY). In both lean and obese humans, injection of exogenous PYY inhibits food intake. A long-acting analogue of GLP-1, exendin-4 is currently being used as an agent for weight control in humans. |
 |
| Immediately after a meal, the stomach may contain up to a liter of material, which will empty slowly into the small intestine. The rate of gastric emptying is dependent on the macronutrient content of the meal and the
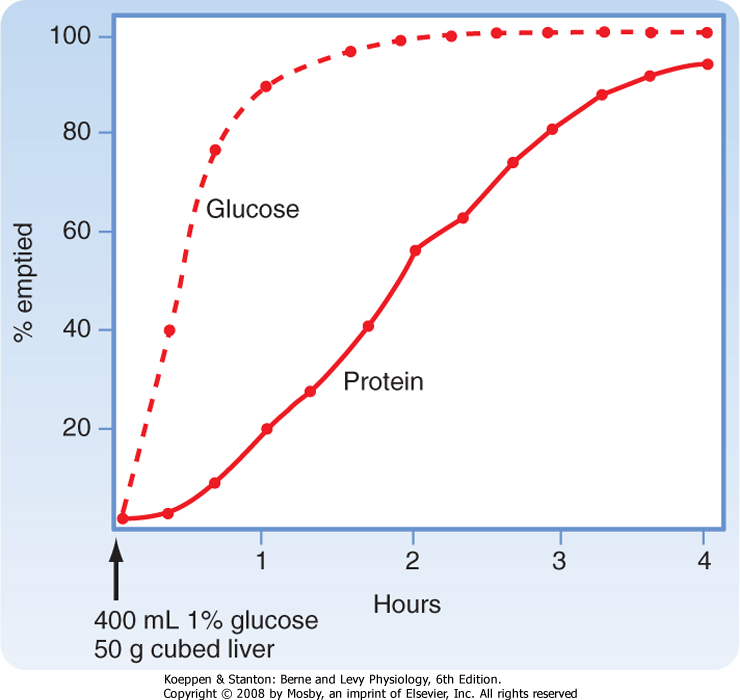
amount of solids contained in the meal. Thus, solids and liquids of similar nutritional composition will empty at different rates. Liquids empty rapidly but solids do so only after a lag phase, which means that after a solid meal, there is a period of time during which little or no emptying occurs (Fig. 29-1).
|
| page 516 |  | | page 517 |
| Figure 29-1 Rates of emptying of different meals from a dog's stomach. A solution (1% glucose) is emptied faster than a digestible solid (cubed liver). Note the lag phase for emptying of the solids, which is related to the time needed to reduce particles below 2 mm in size. (Adapted from Hinder RA, Kelly KA: Am J Physiol 233:E335, 1977.) |
| Regulation of gastric emptying is achieved by alterations in motility of the proximal part of the stomach (fundus and corpus) and distal part of the stomach (pylorus and duodenum). Motor function in these regions is highly coordinated. Recall that during the esophageal and gastric phase of the meal, the predominant reflex response is receptive relaxation. At the same time, peristaltic movements in the more distal part of the stomach (antrum) mix the gastric contents with gastric secretions. The pyloric sphincter is largely closed. Even if it opens periodically, little emptying will occur because the proximal portion of the stomach is relaxed and the antral pump (antral contractions) is not very strong. Subsequently, gastric emptying is brought about by an increase in tone (intraluminal pressure) in the proximal portion of the stomach, increased strength of antral contractions (increased strength of the antral pump), opening of the pylorus to allow the contents to pass, and simultaneous inhibition of duodenal segmental contractions. Liquids and
the semiliquid chyme flow down the pressure gradient from the stomach to the duodenum.
|
| As the meal enters the small intestine, it feeds back via both neural and hormonal pathways to regulate the rate of gastric emptying based on the chemical and physical composition of the chyme. Afferent neurons, predominantly of vagal origin, respond to nutrients, H+, and the hyperosmotic content of chyme as it enters the duodenum. Reflex activation of vagal efferent outflow decreases the strength of antral contractions, contracts the pylorus, and decreases proximal gastric motility (with a decrease in intragastric pressure), thereby resulting in inhibition (slowing) of gastric emptying. This same pathway is probably responsible for the inhibition of gastric acid secretion that occurs when nutrients are in the duodenal lumen. Cholecystokinin (CCK) is released from endocrine cells in the duodenal mucosa in response to such nutrients. This hormone is physiologically important, in addition to its role in neural pathways, in the regulation of gastric emptying, gallbladder contraction, relaxation of the sphincter of Oddi, and pancreatic secretion. Recent experimental evidence suggests that CCK may act as a hormone not only to inhibit gastric emptying but also to stimulate vagal afferent fiber discharge to produce a vagovagal reflex-mediated decrease in gastric emptying.
|
| Surgical treatment of obesity, so-called bariatric surgery, can achieve substantial and lasting weight loss and also help associated health problems such as insulin resistance, hyperlipidemia, and elevated blood pressure. Initially, surgery involved jejunoileal bypass, the removal of a substantial part of the absorptive small intestine, but this procedure is associated with malabsorption and subsequent undesirable sequelae such as diarrhea. |
| The most common surgery that is currently performed in the United States is Roux-en-Y gastric bypass. This procedure involves making a gastric pouch and attaching the jejunum to this pouch. The mechanism by which the procedure is thought to be successful lies in the small size of the gastric pouch, whereby meal size is decreased because of early satiety, and a beneficial effect of the bypass on the profiles of gastrointestinal hormones. |
 |
| How then can gastric emptying proceed in the face of these inhibitory pathways? The amount of chyme in the duodenum decreases as it passes further down the small intestine into the jejunum; thus, the strength of intestinal feedback inhibition fades as there is less activation of the sensory mechanisms in the duodenum by nutrients. At this time, intragastric pressure
in the proximal portion of the stomach increases, thereby moving material into the antrum and toward the antral pump. Antral peristaltic contractions again deepen and culminate in opening of the pylorus and release of gastric contents into the duodenum.
|
| Most of the nutrients ingested by humans are in the chemical form of macromolecules. However, such molecules are too large to be assimilated across the epithelial cells that line the intestinal tract and must therefore be broken down into their smaller constituents by processes of chemical and enzymatic digestion. Secretions arising from the pancreas are quantitatively the largest contributors to enzymatic digestion of the meal. The pancreas also provides additional important secretory products that are vital for normal digestive function. Such products include substances that regulate the function or secretion (or both) of other pancreatic products, as well as water and bicarbonate ions. The latter are involved in neutralizing gastric acid so that the small intestinal lumen has a pH approaching 7.0. This is important because pancreatic enzymes are inactivated by high levels of acidity and also because neutralization of gastric acid reduces the likelihood that the small intestinal mucosa will be injured by such acid acting in combination with pepsin. Quantitatively, the pancreas is the largest contributor to the supply of bicarbonate ions needed to neutralize the gastric acid load, although the biliary ductules and duodenal epithelial cells themselves also contribute.
|
| page 517 |  | | page 518 |
| Pancreatitis can result when enzymes secreted by pancreatic acinar cells become proteolytically activated before they have reached their appropriate site of action in the small intestinal lumen. Indeed, pancreatic juice contains a variety of trypsin inhibitors to reduce the risk of such premature activation because trypsin is the activator of other pro-forms of enzymes secreted in pancreatic juice. A second level of protection lies in the fact that trypsin can itself be degraded by other trypsin molecules. However, in some individuals, pancreatitis still arises spontaneously in the absence of known risk factors, as well as in an inherited pattern. This has been mapped to a specific mutation in trypsin that renders it resistant to degradation by other trypsin molecules. In such individuals, if other defenses have been breached and trypsin becomes active prematurely, a vicious cycle of enzyme activation ensues and bouts of pancreatitis follow. |
 |
|
Table 29-1.
Products of Pancreatic Acinar Cells |
| Precursors of Proteases |
| Trypsinogen |
| Chymotrypsinogen |
| Proelastase |
| Procarboxypeptidase A |
| Procarboxypeptidase B |
| Starch-Digesting Enzymes |
| Amylase |
| Lipid-Digesting Enzymes or Precursors |
| Lipase |
| Nonspecific esterase |
| Prophospholipase A2 |
| Nucleases |
| Deoxyribonuclease |
| Ribonuclease |
| Regulatory Factors |
| Procolipase |
| Trypsin inhibitors |
| Monitor peptide |
| As in the salivary glands, the pancreas has a structure that consists of ducts and acini. The pancreatic acinar cells line the blind ends of a branching ductular system that eventually empties into the main pancreatic duct and from there into the small intestine under
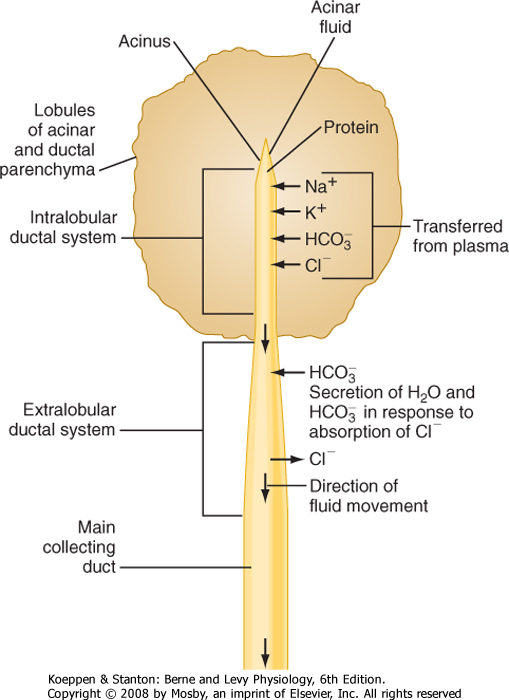
control of the sphincter of Oddi. Also in common with salivary glands, a primary secretion arises in the acini and is then modified as it passes through the pancreatic ducts. In general, the acinar cells supply the organic constituents of the pancreatic juice in a primary secretion whose ionic composition is comparable to that of plasma, whereas the ducts dilute and alkalinize the pancreatic juice while reabsorbing chloride ions (Fig. 29-2). The major constituents of pancreatic juice, which amounts to approximately 1.5 L/day in adult humans, are listed in Table 29-1. This list also outlines the functions of pancreatic secretory products. Many of the digestive enzymes produced by the
pancreas, particularly the proteolytic enzymes, are produced as inactive, precursor forms. Storage in these inactive forms appears to be critically important in preventing the pancreas from digesting itself.
|
| Characteristics and Control of Ductular Secretion
|
| Figure 29-2 Locations of important transport processes involved in the elaboration of pancreatic juice. Acinar fluid is isotonic and resembles plasma in its concentrations of Na+, K+, Cl-, and HCO3-. Secretion of acinar fluid and the proteins that it contains is stimulated primarily by cholecystokinin. The hormone secretin stimulates secretion of water and electrolytes from the cells that line the extralobular ducts. The secretin-stimulated secretion is richer in HCO3- than the acinar secretion because of Cl-/HCO3- exchange. (Adapted from Swanson CH, Solomon AK: J Gen Physiol 62:407, 1973.) |
| page 518 |  | | page 519 |
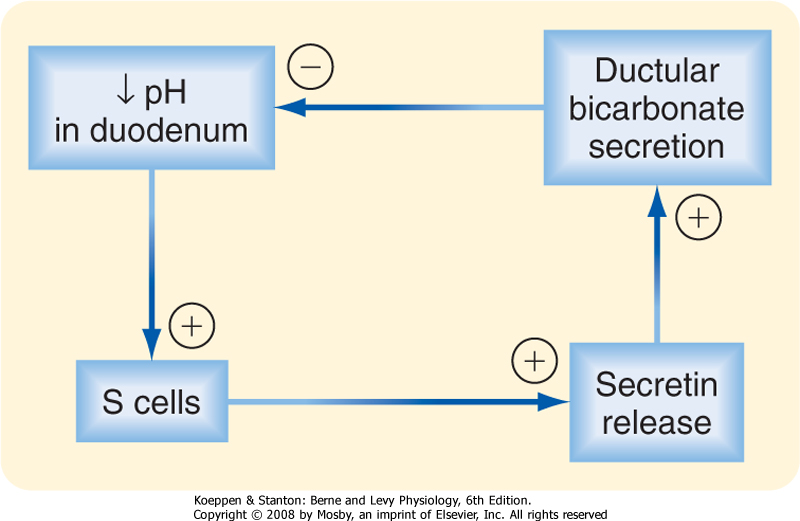
| In this section we consider how the pancreatic ductular cells contribute to the flow and composition of pancreatic juice in the postprandial period. The ducts of the pancreas can be considered the effector arm of a pH regulatory system designed to respond to luminal acid in the small intestine and secrete just enough bicarbonate to restore pH to neutrality (Fig. 29-3). This regulatory function also requires mechanisms to sense luminal pH and convey this information to the pancreas, as well as other epithelia (e.g., biliary ductules and the duodenal epithelium itself) capable of secreting bicarbonate. The pH-sensing mechanism is embodied in specialized endocrine cells localized within the small intestinal epithelium, known as S cells. When luminal pH falls below approximately 4.5, S cells are
triggered to release secretin, presumably in response to protons. The components of this regulatory loop constitute a self-limited system. Thus, as secretin evokes secretion of bicarbonate, pH in the small intestinal lumen will rise and the signal for release of secretin from S cells will be terminated.
|
|
| Figure 29-3 Participation of secretin and HCO3- secretion in a classic negative-feedback loop that responds to a fall in luminal pH in the duodenum. |
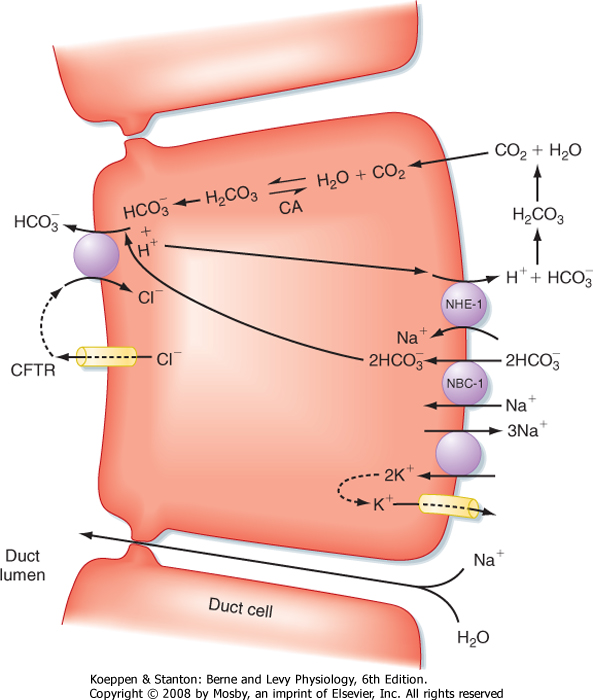
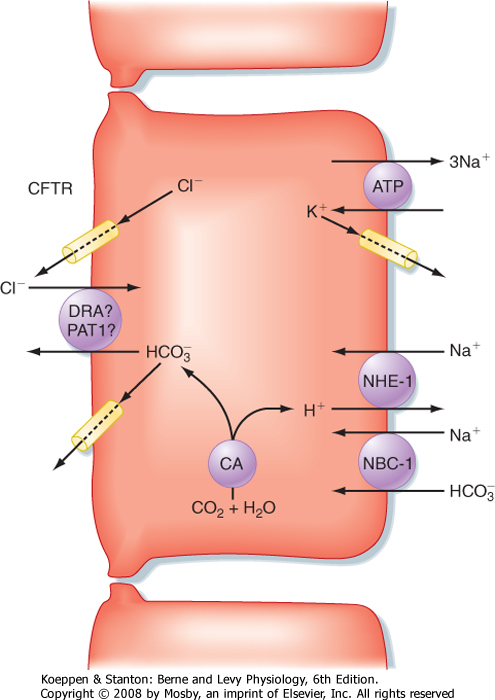
| Figure 29-4 Ion transport pathways in pancreatic duct cells. CA, carbonic anhydrase; CFTR, cystic fibrosis transmembrane conductance regulator; NBC-1, sodium/bicarbonate cotransporter (symporter) type 1; NHE-1, sodium-hydrogen exchanger (antiporter) type 1. |
| At the cellular level, secretin directly stimulates epithelial cells to secrete bicarbonate ions into the ductular lumen, with water following via the paracellular route to maintain osmotic equilibrium. Secretin increases cAMP in the ductular cells and thereby opens CFTR Cl- channels (Fig. 29-4) and causes an outflow of Cl- into the duct lumen. This secondarily
drives the activity of an adjacent antiporter that exchanges the chloride ions for bicarbonate. There is also emerging evidence that CFTR itself may be permeable to some extent to bicarbonate ions when opened. In either case, the bicarbonate secretory process is dependent on CFTR, which provides a rationale for the defects in pancreatic function that are seen in the disease of cystic fibrosis, in which CFTR is mutated. The bicarbonate needed for this secretory process is derived from two sources. Some is taken up across the basolateral membrane of the ductular epithelial cells via the symporter NBC-1 (for sodium-bicarbonate cotransporter type 1). Recall that the process of gastric acid secretion results in an increase in circulating bicarbonate ions, which can serve as a source of bicarbonate to be secreted by the pancreas. However, bicarbonate can also be generated intracellularly via the activity of the enzyme carbonic anhydrase. The net effect is to move HCO3- into the lumen and thereby increase pH and the volume of pancreatic juice.
|
| Characteristics and Control of Acinar Secretion
|
| In contrast to the pancreatic ductules, where secretin is the most important physiological agonist, CCK plays the predominant role at the level of the acinar cells. Thus, it is important to understand how release of CCK is controlled during the small intestinal phase of the response to a meal.
|
| page 519 |  | | page 520 |
| Cystic fibrosis (CF) is a genetic disease that affects the function of a variety of epithelial organs, including the lung, intestine, biliary system, and pancreas. Previously, the disease was almost uniformly fatal during adolescence as a result of severe respiratory infections, but improved antibiotics can now extend life even into the fifth decade or later in some patients. The disease is caused by a mutation in CFTR, which apparently impairs the ability to hydrate and thus alkalinize the luminal contents. In the gastrointestinal system, specifically, this can result in intestinal obstruction, duodenal mucosal injury, and damage to the liver and biliary system, as well as the pancreas. In some patients the endocrine pancreas will be destroyed even before birth, and these patients are stated to be "pancreatic insufficient" and must be given digestive enzyme supplements to maintain adequate levels of nutrient digestion. In other patients with milder mutations, pancreatitis may develop later in life in the absence of other classic CF symptoms, presumably because of failure to wash digestive enzymes out through the pancreatic ducts. In either case, improved recognition and treatment of the pulmonary complications of CF mean that gastrointestinal symptoms, such as liver failure, reduced bile flow, pancreatitis, obstruction, and maldigestion/malabsorption of nutrients, are acquiring increased importance as facets of the disease that must be managed in adults. |
 |
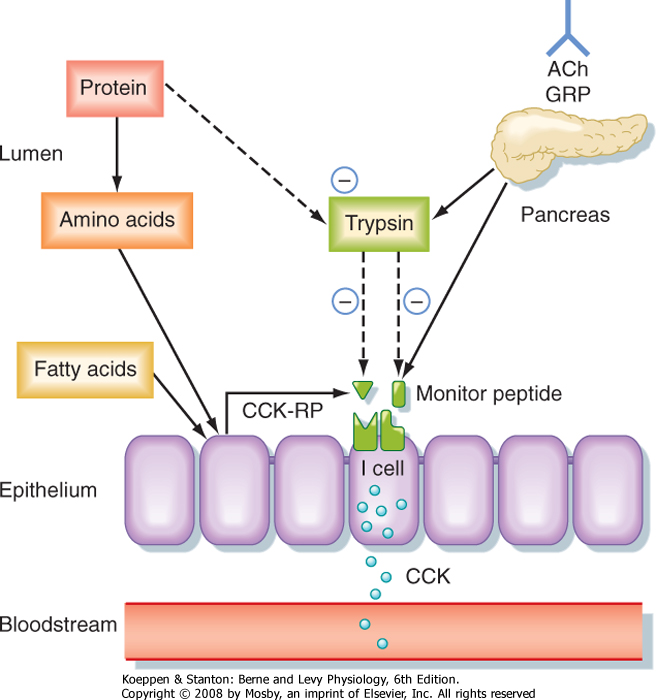
| CCK is the product of I cells, which are also localized to the small intestinal epithelium. These classic enteroendocrine cells release CCK into the interstitial
space when specific food components are present in the lumen, particularly free fatty acids and certain amino acids. Release of CCK from I cells may occur as a result of a direct interaction of fatty acids or amino acids, or both, specifically with the I cells themselves. Release of CCK is also regulated by two luminally acting releasing factors that can stimulate the I cell. The first of these, referred to as CCK-releasing factor (or peptide), is secreted by paracrine cells within the epithelium into the small intestinal lumen, probably in response to products of fat or protein digestion (or both). The second releasing factor, likewise a peptide, is called monitor peptide and is released by pancreatic acinar cells into pancreatic juice. Both CCK-releasing factor and monitor peptide can also be released in response to neural input, which is probably particularly important in initiating pancreatic secretion during the cephalic and gastric phases, thereby preparing the system to digest the meal as soon as it enters the small intestine.
|
|
| Figure 29-5 Mechanisms responsible for controlling the release of cholecystokinin (CCK) from duodenal I cells. ACh, acetylcholine; CCK-RP, CCK-releasing peptide; GRP, gastrin-releasing peptide. Solid arrows represent stimulatory effects, whereas dashed arrows indicate inhibition. (Redrawn from Barrett KE: Gastrointestinal Physiology. New York, McGraw Hill, 2006.) |
| What is the significance of these peptide-releasing factors? Their primary role appears to be to match CCK release, as well as the resulting availability of pancreatic enzymes, to the need for these enzymes to digest the meal in the small intestinal lumen (Fig. 29-5). Because the releasing factors are peptides, they will be subject to proteolytic degradation by enzymes such as pancreatic trypsin in exactly the same way as dietary protein. However, when dietary protein is
ingested, it is present in much greater amounts in the lumen than the releasing factors and thus "competes" with the releasing factors for proteolytic degradation. The net effect is that the releasing factors will be protected from breakdown while the meal is in the small intestine and are therefore available to continue stimulation of CCK release from I cells. However, once the meal has been digested and absorbed, the releasing factors are degraded and the signal for release of CCK is shut off.
|
| CCK evokes secretion by pancreatic acinar cells in two ways. First, it is a classic hormone that travels through the bloodstream to encounter acinar cell CCK1 receptors. However, CCK also stimulates neural reflex pathways that impinge on the pancreas. Vagal afferent nerve endings in the wall of the small intestine are responsive to CCK by virtue of their expression of CCK1 receptors. As described earlier for the effect of CCK on gastric emptying, binding of CCK activates a vagovagal reflex that can further enhance acinar cell secretion via activation of pancreatic enteric neurons and release of a series of neurotransmitters such as acetylcholine, gastrin-releasing peptide, and vasoactive intestinal polypeptide (VIP).
|
| page 520 |  | | page 521 |
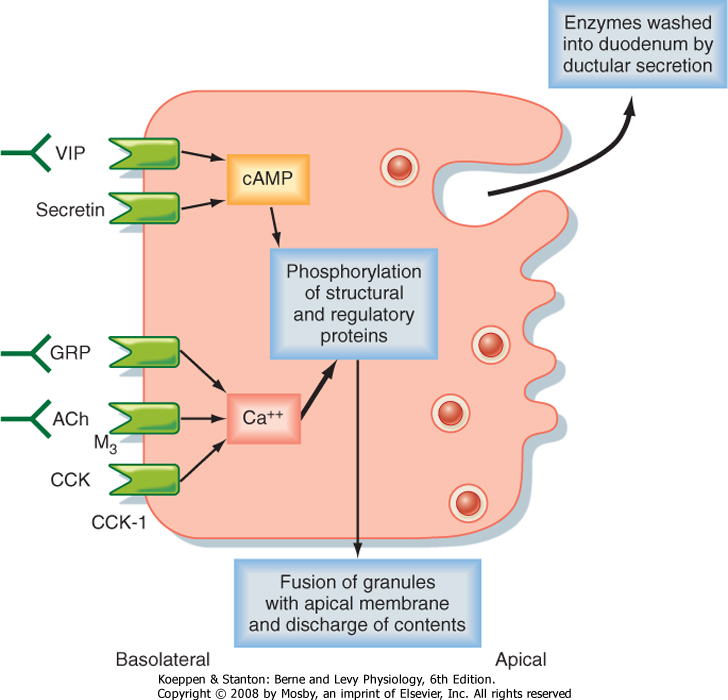
| Figure 29-6 Receptors of the pancreatic acinar cell and regulation of secretion. The block arrow indicates that Ca++-dependent signaling pathways play the most prominent role. ACh, acetylcholine; CCK, cholecystokinin; GRP, gastrin-releasing peptide; VIP, vasoactive intestinal polypeptide. (Redrawn from Barrett KE: Gastrointestinal Physiology. New York, McGraw Hill, 2006.) |
| The secretory products of pancreatic acinar cells are largely presynthesized and stored in granules that cluster toward the apical pole of acinar cells (Fig. 29-6). The most potent stimuli of acinar cell secretion, including CCK itself, acetylcholine, and gastrin-releasing
peptide, act by mobilizing intracellular Ca++. Stimulation of acinar cells results in phosphorylation of a series of regulatory and structural proteins within the cell cytosol that serve to move the granules closer to the apical membrane, where fusion of granule and plasma membranes can occur. The contents of the granule are then discharged into the acinar lumen and subsequently washed out of the pancreas by an exudate of plasma crossing the tight junctions linking the acinar cells together and ultimately by ductular secretions. In the period between meals, in contrast, the granule constituents are resynthesized by the acinar cells and then stored until needed to digest the next meal. The signals that mediate granule resynthesis are less well understood, but resynthesis may be stimulated by the same agonists that evoke the initial secretory response.
|
| Another important digestive juice that is mixed with the meal when it is present in the small intestinal lumen is bile. Bile is produced by the liver, and the mechanisms that are involved, as well as the specific constituents, will be discussed in greater detail in Chapter 31 when we address the transport and metabolic functions of the liver. However, for purposes of the current discussion, bile is a secretion that serves to aid in the digestion and absorption of lipids. Bile flowing out of the liver is stored and concentrated in the gallbladder until it is released in response to ingestion of a meal. Contraction of the gallbladder, as well as relaxation of the sphincter of Oddi, are evoked predominantly by CCK. Indeed, its ability to contract the gallbladder gave CCK its name.
|
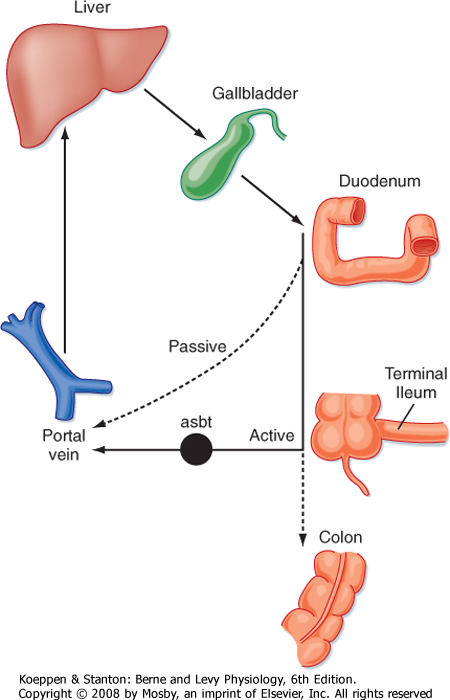
| When considering the small intestinal phase of meal assimilation, the bile constituents that we are most concerned with are the bile acids. These acids form structures known as micelles that serve to shield the hydrophobic products of lipid digestion from the aqueous environment of the lumen. Bile acids are in essence biological detergents, and large quantities are needed on a daily basis for optimal lipid absorption-as much as 1 to 2 g/day. The majority of the bile acid pool is recycled from the intestine back to the liver after each meal via the enterohepatic circulation (Fig. 29-7). Thus, bile acids are synthesized in a conjugated form that limits their ability to passively cross the epithelium lining the intestine so that they are retained in the lumen to participate in lipid assimilation (see later). However, when the meal contents reach the terminal ileum, after lipid absorption has been completed, the conjugated bile acids are reabsorbed by a symporter that specifically takes up conjugated bile acids in association with sodium ions, known as the apical Na+-dependent bile acid transporter (asbt). Only a minor portion of the bile acid pool is left to spill over into the colon in health, and here bile acids become deconjugated and subject to passive reabsorption (Fig. 29-7). The net effect is to cycle the majority of the bile acid pool between the liver and intestine on a daily basis, coincident with signals arising in the postprandial period. For example, CCK is a potent agonist of gallbladder contraction.
|
| page 521 |  | | page 522 |
| CARBOHYDRATE ASSIMILATION
|
| Figure 29-7 Enterohepatic circulation of bile acids. |
| Of course, the most important physiological function of the small intestine is to take up the products of digestion of ingested nutrients. Quantitatively, the most significant nutrients (macronutrients) fall into three classes: carbohydrates, proteins, and lipids. The small intestine is critical not only for absorption of such nutrients into the body but also for the final stages of their digestion into molecules that are simple enough to be transported across the intestinal epithelium.
We will consider the processes involved in the assimilation of each of these nutrients in turn, beginning with carbohydrates. Carbohydrate digestion occurs in two phases: in the lumen of the intestine and then on the surface of enterocytes in a process known as brush border digestion. The latter is thought to be important in generating simple, absorbable sugars only at the point where they can finally be absorbed. This may therefore limit their exposure to the small number of bacteria present in the small intestinal lumen that might otherwise use these sugars as nutrients.
|
| Digestion of Carbohydrates
|
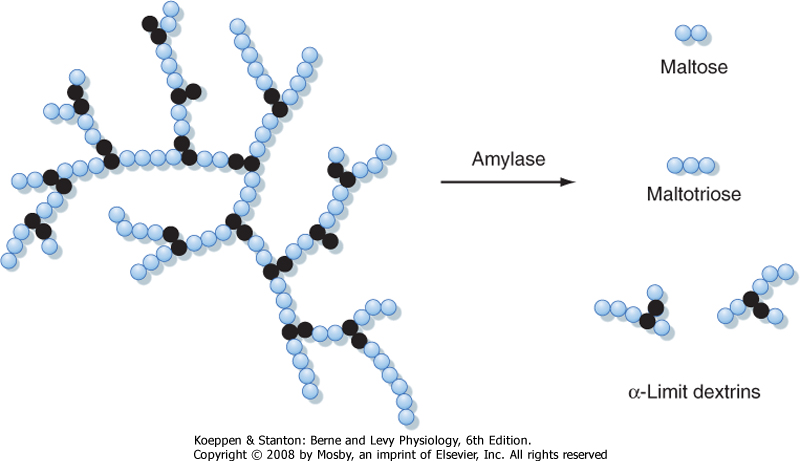
| Dietary carbohydrates are composed of several different molecular classes. Starch, the first of these, is a mixture of both straight- and branched-chain polymers of glucose. The straight-chain polymers are called amylose and the branched-chain molecules are called amylopectin (Fig. 29-8). Starch is a particularly important source of calories, especially in developing countries, and is found predominantly in cereal products. Disaccharides are a second class of carbohydrate nutrients that includes sucrose (consisting of glucose and fructose) and lactose (consisting of glucose and galactose), which is an important caloric source in infants. It is, however, a key principle that the intestine can absorb only monosaccharides and not larger carbohydrates. Finally, many food items of vegetable origin contain dietary fiber, which consists of carbohydrate polymers that cannot be digested by human enzymes. These polymers are instead digested by bacteria present largely in the colonic lumen (see Chapter 30), thereby allowing salvage of their caloric value.
|
|
| Figure 29-8 Structure of amylopectin and the action of amylase. The colored circles represent glucose monomers linked by α-1,4 bonds. The black circles represent glucose units linked by α-1,6 bonds at the branch points. |
| page 522 |  | | page 523 |
|
Table 29-2.
Brush Border Carbohydrate Hydrolases |
| Enzyme | Specificity/Substrates | Products |
| Sucrase | α-1,4 bonds of maltose, maltotriose, and sucrose | Glucose, fructose |
| Isomaltase | α-1,4 bonds of maltose, maltotriose; α-1,6 bonds of α-limit dextrins | Glucose |
| Glucoamylase | α-1,4 bonds of maltose, maltotriose | Glucose |
| Lactase | Lactose | Glucose, galactose |
| Lactose intolerance is relatively common in adults from specific ethnic groups, such as Asians, African Americans, and Hispanics. The disorder reflects a normal developmental decline in the expression of lactase by enterocytes, particularly when lactose is not a consistent component of the diet. In such individuals, consumption of foods containing large quantities of lactose, such as milk and ice cream, can result in abdominal cramping, gas, and diarrhea. These symptoms reflect a relative inability to digest lactose; thus it remains in the lumen and water is retained. Some patients are benefited by administration of a bacterially derived lactase enzyme given in tablet form, before the ingestion of dairy products. |
 |
| Dietary disaccharides are hydrolyzed to their component monomers directly on the surface of small intestinal epithelial cells in a process known as brush border digestion, mediated by a family of membrane-bound, heavily glycosylated hydrolytic enzymes synthesized by small intestinal epithelial cells. Brush border hydrolases critical to the digestion of dietary carbohydrates include sucrase, isomaltase, glucoamylase, and lactase (Table 29-2). Glycosylation of these
hydrolases is believed to protect them to some extent from degradation by luminal pancreatic proteases. However, between meals, the hydrolases are degraded and must therefore be resynthesized by the enterocyte to participate in digesting the next carbohydrate meal. Sucrase/isomaltase and glucoamylase are synthesized in quantities that are in excess of requirements, and assimilation of their products into the body is limited by the availability of specific membrane transporters for these monosaccharides, as discussed later. Lactase, in contrast, shows a developmental decline in expression after weaning. The relative paucity of lactase means that digestion of lactose, rather than uptake of the resulting products, is rate limiting for assimilation. If lactase levels fall below a certain threshold, the disease of lactose intolerance results.
|
|
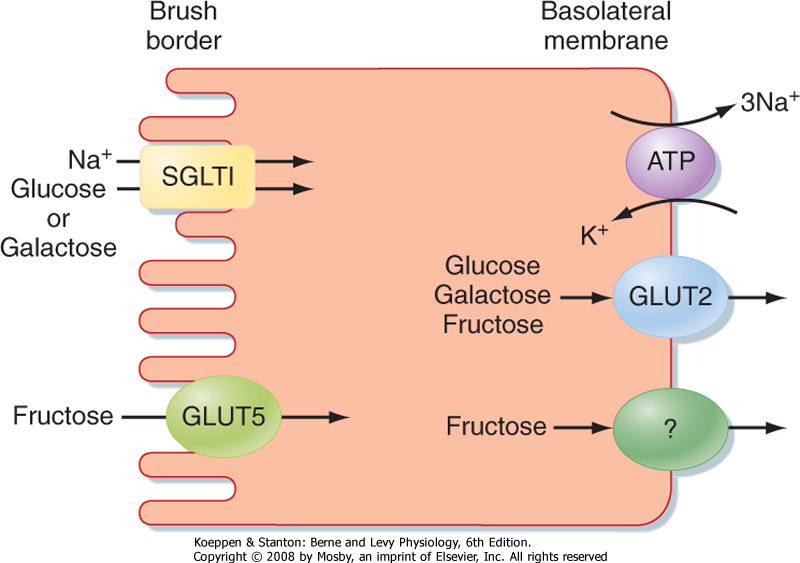
| Figure 29-9 Absorption of glucose, galactose, and fructose in the small intestine. |
| Digestion of starch occurs in two phases. The first takes place in the lumen and is actually initiated in the oral cavity via the activity of salivary amylase, as discussed in Chapter 27. Salivary amylase, however, is not essential for starch digestion, although it may assume greater importance in neonates or patients in whom the output of pancreatic enzymes is impaired by disease. Quantitatively, the most significant contributor to the luminal digestion of starch is pancreatic amylase. Both enzymes hydrolyze internal α-1,4 bonds in both amylose and amylopectin, but not external
bonds nor the α-1,6 bonds that form the branch points in the amylopectin molecule (Fig. 29-8). Thus, digestion of starch by amylase is of necessity incomplete and results in short oligomers of glucose, including dimers (maltose) and trimers (maltotriose), as well as the simplest branching structures, which are called α-limit dextrins. Thus, to allow absorption of its constituent monosaccharides, starch must also undergo brush border digestion.
|
| At the brush border, straight-chain glucose oligomers can be digested by the hydrolases glucoamylase, sucrase, or isomaltase (Table 29-2). All yield free glucose monomers, which can then be absorbed by the mechanisms discussed later. For α-limit dextrins, on the other hand, isomaltase activity is critical because it is the only enzyme that can cleave not only α-1,4 bonds but also the α-1,6 bonds that make up the branch points.
|
| Water-soluble monosaccharides resulting from digestion must next be transported across the hydrophobic membrane of the enterocyte. The sodium/glucose transporter 1 (SGLT1) is a symporter that takes up glucose (and galactose) against its concentration gradient by coupling its transport to that of Na+ (Fig. 29-9). Once inside the cytosol, glucose and galactose can be retained for the epithelium's metabolic needs or can exit the cell across its basolateral pole via a transporter known as GLUT2. Fructose, in contrast, is taken up across the apical membrane by GLUT5. However, because fructose transport is not coupled to that of Na+, its uptake is relatively inefficient and can easily be overwhelmed if large quantities of food containing this sugar are ingested. The symptoms that occur from this malabsorption are similar to those experienced by a lactose-intolerant patient who consumes lactose.
|
| page 523 |  | | page 524 |
| A rare genetic disorder results in an inability of the intestine to absorb glucose or galactose. This disease state has been mapped to a variety of mutations in the SGLT1 gene that result in a faulty or unexpressed protein or, more commonly, failure of the protein to traffic appropriately to the apical membrane of enterocytes. In patients carrying such mutations, malabsorbed glucose contributes to diarrheal and other symptoms, as discussed earlier for lactose intolerance. Despite the rarity of the disease, it is important in terms of the insight that it has provided into a critical process of intestinal epithelial transport. Finally, additional, milder mutations in SGLT1 that reduce transport activity of the protein only partially may nevertheless account for gastrointestinal symptoms and have been implicated in at least some cases of irritable bowel syndrome. |
 |
|
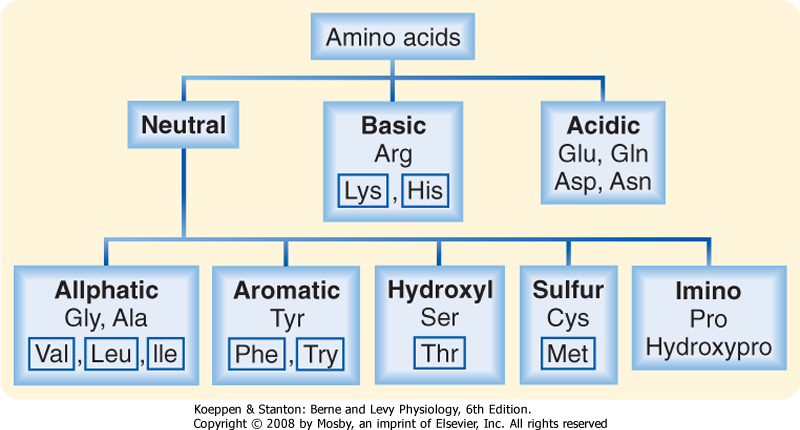
| Figure 29-10 Naturally occurring dietary amino acids. Those in boxes are essential amino acids that cannot be synthesized by humans and thus must be obtained from the diet. (Redrawn from Barrett KE: Gastrointestinal Physiology. New York, McGraw Hill, 2006.) |
| Proteins are also water-soluble polymers that must be digested into their smaller constituents before absorption is possible. Their absorption is more complicated
than that of carbohydrates because they contain 20 different amino acids and short oligomers of these amino acids (dipeptides, tripeptides, and perhaps even tetrapeptides) can also be transported by enterocytes. The body, particularly the liver (see Chapter 31), has substantial ability to interconvert various amino acids subject to the body's needs. However, some amino acids, termed the essential amino acids, cannot be synthesized by the body either de novo or from other amino acids and thus must be obtained from the diet. The amino acids that must be obtained in this way in humans are designated in Figure 29-10.
|
| Proteins can be hydrolyzed to long peptides simply by virtue of the acidic pH that exists in the gastric lumen. However, for assimilation of proteins into the body, three phases of enzymatically mediated digestion are required (Fig. 29-11). Like acid hydrolysis, the first of these phases takes place in the gastric lumen and is mediated by pepsin, the product of chief cells localized to the gastric glands. When gastric secretion is activated by signals coincident with the ingestion of a meal, pepsin is released from the chief cells as the inactive precursor pepsinogen. At acidic pH, this precursor is autocatalytically cleaved to yield the active enzyme. Pepsin is highly specialized to act in the stomach in that it is activated rather than inhibited by low pH. The enzyme cleaves proteins at sites of neutral amino acids, with a preference for aromatic or large aliphatic side chains. Because such amino acids occur only relatively infrequently in a given protein, pepsin is not capable of digesting protein fully into a form that can be absorbed by the intestine and instead yields a mixture of intact protein, large peptides (the majority), and a limited number of free amino acids.
|
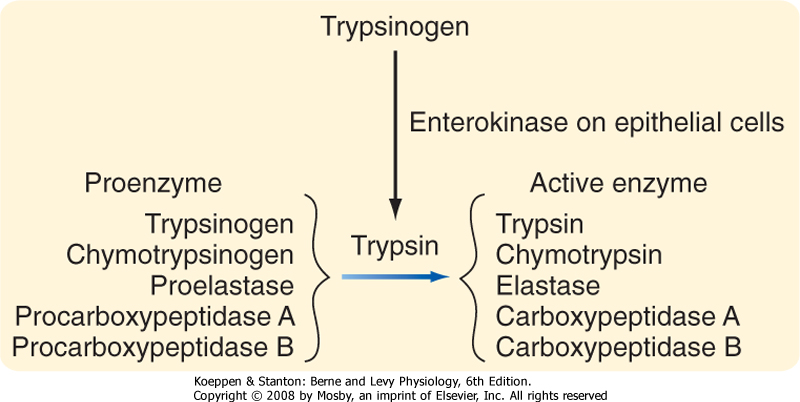
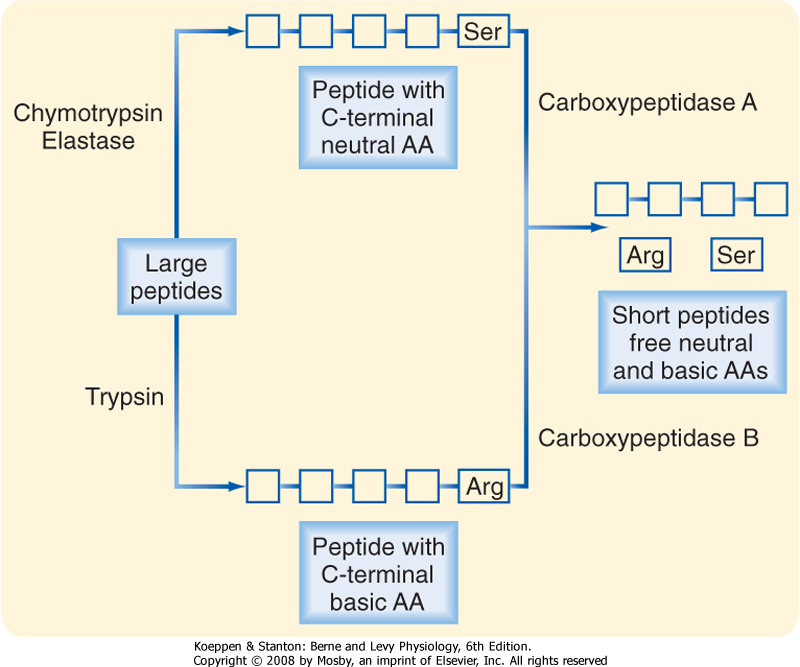
| On moving into the small intestine, the partially digested protein next encounters the proteases provided in pancreatic juice. Recall that these enzymes are secreted in inactive forms. How then are they activated to begin the process of protein digestion? In fact, activation of protease is delayed until these enzymes are in the lumen by virtue of the localized presence of an activating enzyme, enterokinase, only on the brush border of small intestinal epithelial cells (Fig. 29-12). Enterokinase cleaves trypsinogen to yield active trypsin. Trypsin, in turn, is capable of cleaving all the other protease precursors secreted by the pancreas, thereby resulting in a mixture of enzymes that can almost completely digest the vast majority of dietary proteins. Trypsin is a so-called endopeptidase that is capable of cleaving such proteins only at internal bonds within the peptide chain rather than releasing individual amino acids from the end of the chain. Trypsin is specific for cleavage at basic amino acids, and such cleavage results in a set of shorter peptides with a basic amino acid at their C-terminus. The two other pancreatic endopeptidases, chymotrypsin and elastase, on the other hand, have a similar mechanism of action but cleave at sites of neutral amino acids. The peptides that result from endopeptidase activity are then acted on by pancreatic ectopeptidases. These enzymes cleave single amino acids from the end of a peptide chain, and those present in pancreatic juice are specific for either neutral (carboxypeptidase A) or basic (carboxypeptidase B) amino acids situated at the C-terminus. Thus, the products that result overall after digestion of a protein meal by gastric and pancreatic secretions include neutral and basic amino acids, as well as short peptides that have acidic amino acids at their C-termini and thus are resistant to carboxypeptidase A or B (Fig. 29-13).
|
| page 524 |  | | page 525 |
| Figure 29-11 Hierarchy of proteases and peptidases that function in the stomach and small intestine to digest dietary protein. Proteins are absorbed as either single amino acids (70%) or short peptides (30%). (Adapted from Van Dyke RW: In Sleisenger MH, Fordtran JS [eds]: Gastrointestinal Disease, 4th ed. Philadelphia, Saunders, 1989.) |
| Figure 29-12 Conversion of the inactive proenzymes of pancreatic juice to active enzymes by the action of trypsin. Trypsinogen in pancreatic juice is proteolytically converted to active trypsin by enterokinase expressed on the surface of epithelial cells of the duodenum and jejunum. Trypsin then activates the other proenzymes as shown. |
| Figure 29-13 Luminal digestion of peptides resulting from partial proteolysis in the stomach. AA, amino acid. (Redrawn from Barrett KE: Gastrointestinal Physiology. New York, McGraw Hill, 2006.) |
| page 525 |  | | page 526 |
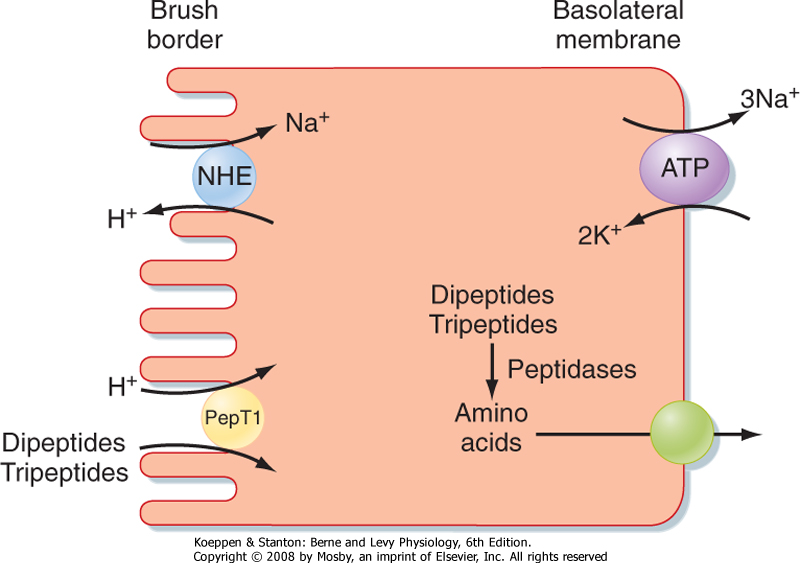
| Figure 29-14 A wide variety of dipeptides and tripeptides are taken up across the brush border membrane by the proton-coupled symporter known as PepT1. The proton gradient is created by the action of sodium/hydrogen exchangers (NHEs) in the apical membrane. |
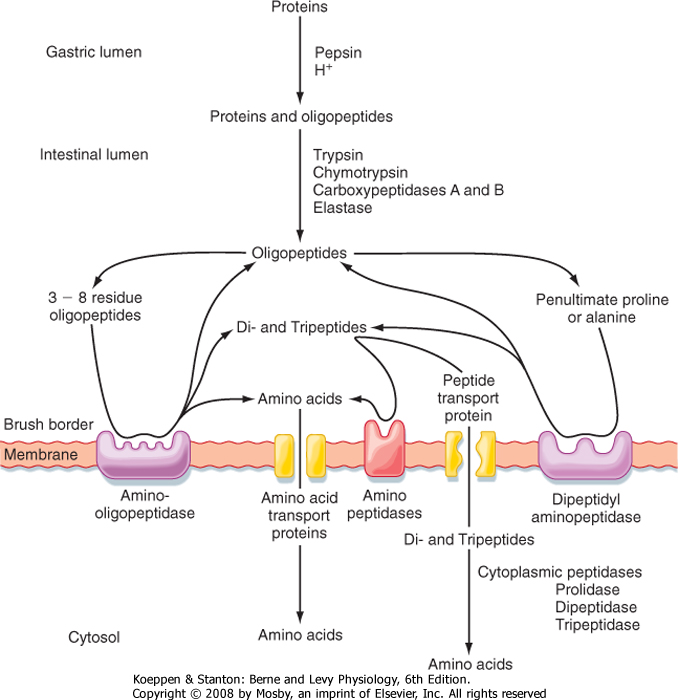
| The final phase of protein digestion then takes place at the brush border. Mature enterocytes express a variety of peptidases on their brush borders, including both aminopeptidases and carboxypeptidases, that generate products suitable for uptake across the apical membrane (Fig. 29-11). However, it should be noted that even with the substantial complement of active proteolytic enzymes, some dietary peptides are either relatively or totally resistant to hydrolysis. In particular, peptides containing either proline or glycine are
digested very slowly. Fortunately, the intestine can take up not only single amino acids but also short peptides. Peptides that are taken up into the enterocyte in their intact form are then subjected to a final stage of digestion in the cytosol of the enterocyte to liberate their constituent amino acids for use in the cell or elsewhere in the body (Fig. 29-14).
|
| UPTAKE OF PEPTIDES AND AMINO ACIDS
|
| The body is also endowed with a series of membrane transporters capable of promoting uptake of the water-soluble products of protein digestion. Given the large number of amino acids, there is a relatively large number of specific transporters (Figs. 29-11 and 29-14). Amino acid transporters are of clinical interest because their absence in a variety of genetic disorders results in a diminished ability to transport the relevant amino acid or acids. However, such mutations are often clinically silent, at least from a nutritional standpoint, because the amino acid in question can be assimilated by other transporters with overlapping specificity or in the form of peptides. This does not rule out the possibility of pathology in other organ systems in which the transporter of interest may normally be expressed (e.g., cysteinuria). In general, amino acid transporters have reasonably broad specificity and usually carry a subset of possible amino acids (e.g., neutral, anionic, or cationic), but with some overlap in their affinity for particular amino acids. Furthermore, some (but not all) of the amino acid transporters are symporters that carry their substrate amino acids in conjunction with obligatory uptake of Na+.
|
| The redundancy in uptake mechanisms for the products of protein digestion underscores the importance of this process and also means that deficiencies in specific amino acid assimilation across the intestine are relatively rare. However, under certain circumstances, mutations in proteins responsible for specific amino acid transport can lead to pathology in other organs. One example is the disease of cysteinuria, which is a molecularly heterogeneous disease involving mutations in a variety of amino acid transporters capable of transporting cysteine. Because cysteine can also be assimilated across the gut in the form of peptides, nutritional deficiencies do not occur despite a lack of intestinal uptake mechanisms for this particular amino acid. In contrast, cysteine can only be poorly reabsorbed from the urine of patients suffering from cysteinuria, and kidney stones can form because this amino acid is relatively insoluble. Pathophysiology can likewise arise secondary to mutations in SLC6A19, a Na+-independent transporter of neutral amino acids, and result in a condition known as Hartnup's disease. Again, nutritional deficiencies are relatively rare, but such patients may lose neutral amino acids in urine and display symptoms related to the importance of such amino acids in the brain and skin. |
 |
| The small intestine is also notable for its ability to take up short peptides (Fig. 29-14). The primary transporter responsible for such uptake is called PepT1 (for peptide transporter 1) and is a symporter that carries
peptides in conjunction with protons. Peptides taken up into enterocytes are then immediately hydrolyzed by a series of cytosolic peptidases into their constituent amino acids. Amino acids not required by the enterocyte are in turn exported across the basolateral membrane and enter blood capillaries to be transported to the liver via the portal vein. PepT1 is also of clinical interest because it can mediate the uptake of so-called peptidomimetic drugs, which include a variety of antibiotics, as well as cancer chemotherapeutic agents. The mechanisms by which amino acids and peptidomimetic drugs exit the enterocyte are not fully understood but are presumed to involve additional transport proteins.
|
| Lipids, defined as substances that are more soluble in organic solvents than in water, are the third major class of macronutrients making up the human diet. Lipids supply more significant calories on a per-gram basis than proteins or carbohydrates do and are thus of major nutritional significance, as well as having a propensity to contribute to obesity if consumed in excessive amounts. Lipids also dissolve volatile compounds that contribute to the food's taste and aroma.
|
| page 526 |  | | page 527 |
| The predominant form of lipid in the human diet is triglyceride, found in oils and other fats. The majority of these triglycerides have long-chain fatty acids (carbon chains longer than 12 carbons) esterified to
the glycerol backbone. Additional lipid is supplied in the form of phospholipids and cholesterol, mostly arising from cell membranes. It is also important to consider that the intestine is presented daily not only with dietary lipid but also with lipid originating from the liver in biliary secretions, as described in more detail in Chapter 31. Indeed, the cholesterol supplied in bile exceeds that provided in the diet on a daily basis in all but the most egg-loving individuals. Finally, though present in only trace amounts, the fat-soluble vitamins (A, D, E and K) are essential nutrients that should be supplied in the diet to avoid disease. These substances are almost entirely insoluble in water and thus require special handling to promote their uptake into the body.
|
| Emulsification and Solubilization of Lipids
|
| When a fatty meal is ingested, the lipid becomes liquefied at body temperature and floats on the surface of the gastric contents. This would limit the area of the interface between the aqueous and lipid phases of the gastric contents and thus restrict access of enzymes capable of breaking down the lipid to forms that can be absorbed because the lipolytic enzymes, as proteins, reside in the aqueous phase. Therefore, an early stage in the assimilation of lipid is its emulsification. The mixing action of the stomach churns the dietary lipid into a suspension of fine droplets, which vastly increases the surface area of the lipid phase.
|
| Lipid absorption is also facilitated by the formation of a micellar solution with the aid of bile acids supplied in biliary secretions. Details of this process will be discussed subsequently.
|
| Lipid digestion begins in the stomach. Gastric lipase is released in large quantities from gastric chief cells; it adsorbs to the surface of fat droplets dispersed in the gastric contents and hydrolyzes component triglycerides to diglycerides and free fatty acids. However, little lipid assimilation can take place in the stomach because of the acidic pH of the lumen, which results in protonation of the free fatty acids released by gastric lipase. Lipolysis is also incomplete in the stomach because gastric lipase, despite its optimum catalytic activity at acidic pH, is not capable of hydrolyzing the second position of the triglyceride ester, which means that the molecule cannot be fully broken down into components that can be absorbed into the body. There is also little if any breakdown of cholesterol esters or the esters of fat-soluble vitamins. Indeed, gastric lipolysis is dispensable in healthy individuals because of the marked excess of pancreatic enzymes.
|
| The majority of lipolysis takes place in the small intestine in health. Pancreatic juice contains three important lipolytic enzymes that are optimized for activity at neutral pH. The first of these is pancreatic lipase. This enzyme differs from the stomach enzyme in that it is capable of hydrolyzing both the 1 and 2 positions of triglyceride to yield a large quantity of free fatty acids and monoglycerides. At neutral pH, the head groups of the free fatty acids are charged, and thus these molecules migrate to the surface of the oil droplets. Lipase also displays an apparent paradox in that it is inhibited by bile acids, which also form part of the small intestinal contents. Bile acids adsorb to the surface of the oil droplets and would thereby cause lipase to dissociate. However, lipase activity is sustained by an important cofactor, colipase, which is also supplied in pancreatic juice. Colipase is a bridging molecule that binds both to bile acids and to lipase; it anchors lipase to the oil droplet even in the presence of bile acids.
|
| Pancreatic juice also contains two additional enzymes that are important in fat digestion. The first of these is phospholipase A2, which hydrolyzes phospholipids such as those present in cell membranes. Predictably, this enzyme would be quite toxic in the absence of dietary substrates, and thus it is secreted as an inactive pro-form that is activated only when it reaches the small intestine. Furthermore, pancreatic juice contains a relatively nonspecific, so-called cholesterol esterase that can break down not only esters of cholesterol, as its name implies, but also esters of fat-soluble vitamins and even triglycerides. Interestingly, this enzyme requires bile acids for activity (contrast with lipase, discussed earlier), and it is related to an enzyme produced in breast milk that plays an important role in lipolysis in neonates.
|
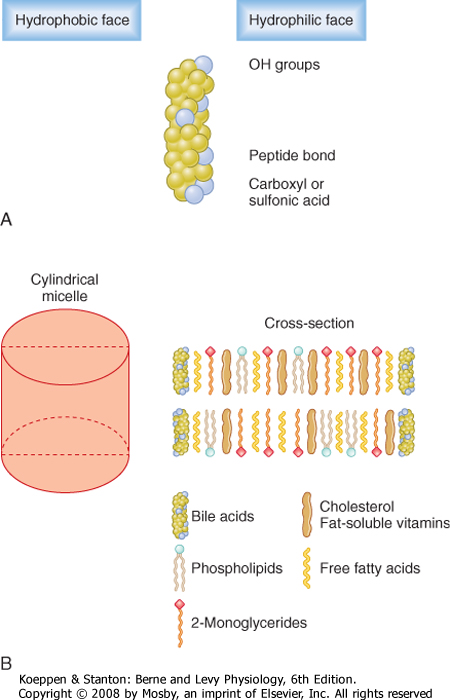
| As lipolysis proceeds, the products are abstracted from the lipid droplet, first into a lamellar, or membrane, phase and subsequently into mixed micelles composed of lipolytic products, as well as bile acids. The amphipathic (meaning that they have both a hydrophobic and hydrophilic face) bile acids serve to shield the hydrophobic regions of lipolytic products from water while presenting their own hydrophilic faces to the aqueous environment (Fig. 29-15). Micelles are truly in solution and thus markedly increase the solubility of lipid in the intestinal contents. This increases the rate at which molecules such as fatty acids can diffuse to the absorptive epithelial surface. Nevertheless, given the very large surface area of the small intestine and the appreciable solubility of the products of triglyceride hydrolysis, micelles are not essential for the absorption of triglyceride. Thus, patients who have insufficient output of bile acids (caused, for example, by a gallstone that obstructs bile output) do not normally show fat malabsorption. On the other hand, cholesterol and the fat-soluble vitamins are almost totally insoluble in water and accordingly require micelles to be absorbed even after they have been digested. Thus, if luminal bile acid concentrations fall below the critical micellar concentration, patients can become deficient in fat-soluble vitamins.
|
| Uptake of Lipids and Subsequent Handling
|
| page 527 |  | | page 528 |
| Figure 29-15 Schematic depiction of bile acids (A) and mixed micelles (B). Bile acids in solution are amphipathic. Mixed micelles are cylindrical assemblages of bile acids with other dietary lipids. |
| A relatively new treatment of hypercholesterolemia targets absorption of cholesterol, either derived from the diet or in bile, across the small intestinal epithelium. Ezetimibe is a drug that specifically blocks uptake of cholesterol by inhibiting the activity of the NPC1L1 protein expressed in the apical membrane of enterocytes. In conjunction with other drugs designed to counter atherosclerosis, this may be a useful adjunct in that it can interrupt the enterohepatic circulation, as well as prevent the absorption of dietary cholesterol. Clinical studies suggest that ezetimibe may synergistically improve the efficacy of other strategies designed to reduce circulating levels of low-density lipoprotein cholesterol in those at risk for cardiovascular disease. |
 |
| The products of fat digestion are believed to be capable of crossing cell membranes readily because of their lipophilicity. However, recent evidence suggests that their uptake may alternatively or additionally be regulated via the activity of specific membrane transporters. A microvillus membrane fatty acid-binding protein (MVM-FABP) provides for the uptake of long-chain fatty acids across the brush border. Likewise, Niemann
Pick C1 like 1 (NPC1L1) has recently been identified as an uptake pathway for cholesterol and may be a therapeutic target in patients who suffer from pathological increases in circulating cholesterol (hypercholesterolemia). However, uptake of cholesterol overall is relatively inefficient because this molecule, along with plant sterols, can also be actively effluxed from enterocytes back into the cytosol by a heterodimeric complex of two so-called "ABC" (ATP-binding cassette) transporters termed ABC G5 and G8.
|
| Lipids also differ from carbohydrates and proteins in terms of their fate after absorption into the enterocyte. Unlike monosaccharides and amino acids, which leave the enterocyte in molecular form and enter the portal circulation, the products of lipolysis are reesterified in the enterocyte to form triglycerides, phospholipids, and cholesterol esters. These metabolic events take place in the smooth endoplasmic reticulum. Concurrently, the enterocyte synthesizes a series of proteins known as apolipoproteins in the rough endoplasmic reticulum. These proteins are then combined with the resynthesized lipids to form a structure known as a chylomicron, which consists of a lipid core (predominantly triglyceride with much less cholesterol, phospholipid, and fat-soluble vitamin esters) coated by the apolipoproteins. The chylomicrons are then exported from the enterocyte by a process of exocytosis. However, on entering the lamina propria, they are too large (approximately 750 to 5000 Å in diameter) to permeate through the intercellular spaces of the mucosal capillaries. Instead, they are taken up into lymphatics in the lamina propria and as such bypass the portal circulation and, at least for their first pass, the liver. Eventually, chylomicrons in the lymph enter the bloodstream via the thoracic duct and then serve as the vehicle to transport lipids around the body for use by cells in other organs. The only exception to this chylomicron-mediated transport is for medium-chain fatty acids. These acids are relatively water soluble and can also permeate enterocyte tight junctions appreciably, which means that they bypass the intracellular processing steps described earlier and are not packaged into chylomicrons. They therefore enter the portal circulation and are more readily available to other tissues. A diet rich in medium-chain triglycerides may be of particular benefit in patients with inadequate bile acid pools.
|
| WATER AND ELECTROLYTE SECRETION AND ABSORPTION
|
| page 528 |  | | page 529 |
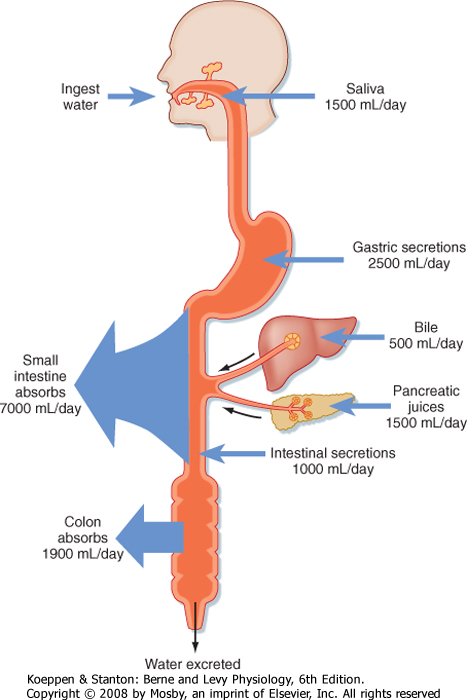
| Figure 29-16 Overall fluid balance in the human gastrointestinal tract. About 2 L of water is ingested and 7 L of various secretions enters the gastrointestinal tract. Of this total, most is absorbed in the small intestine. About 2 L is passed onto the colon, the vast majority of which is absorbed in health. (From Vander AJ et al: Human Physiology, 6th ed. New York, McGraw Hill, 1994.) |
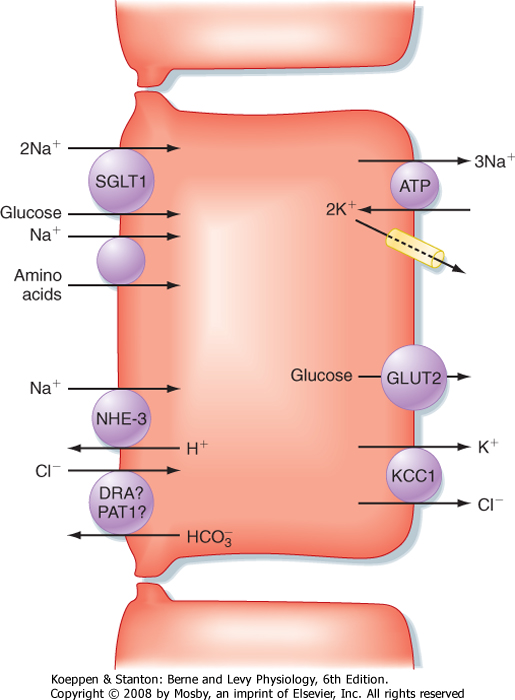
| Figure 29-17 Mechanisms of NaCl absorption in the small intestine. |
| The foregoing description of digestion has stressed that these processes take place in the small intestine in an aqueous milieu. The fluidity of intestinal contents, especially in the small intestine, is important in allowing the meal to be propelled along the length of the intestine and to permit digested nutrients to diffuse to their site of absorption. Part of this fluid is derived from oral intake, but in most adults this consists of only about 1 or 2 L/day derived from both food and drink (Fig. 29-16). Additional fluid is supplied by the stomach and the small intestine themselves, as well as
the organs that drain into the gastrointestinal tract. In total, these secretions add another 8 L, which means that the intestine is presented with approximately 9 L of fluid on a daily basis. However, in health only about 2 L of this load is passed to the colon for reabsorption, and eventually only 100 to 200 mL exits in stool. Thus, the transport vector for fluid throughout the intestine emphasizes absorption. During the postprandial period, such absorption is promoted in the small intestine predominantly via the osmotic effects of nutrient absorption. An osmotic gradient is established across the intestinal epithelium that simultaneously drives the movement of water across the tight junctions. The generic mechanism for nutrient-driven Na+ and water absorption in the small intestine is diagrammed in Figure 29-17. Moreover, in the period between meals, when nutrients are absent, fluid absorption can still occur via the coupled uptake of Na+ and Cl- mediated
by the cooperative interaction of the NHE-3 Na+-H+ antiporter and a Cl--HCO3- antiporter (see Fig. 29-17).
|
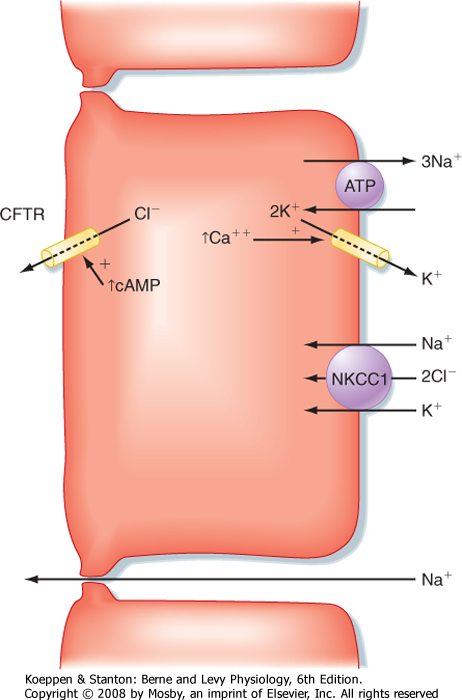
| Even though net water and electrolyte transport in the small intestine predominantly follows an absorptive vector, this does not imply that the tissue fails to participate in electrolyte secretion. Secretion is regulated in response to signals derived from the luminal contents and in response to deformation of the mucosa or intestinal distention, or both. Critical secretagogues include acetylcholine, VIP, prostaglandins, and serotonin. Secretion makes sure that the intestinal contents are appropriately fluid while digestion and absorption are still ongoing and may be important to lubricate the passage of food particles along the length of the intestine. For example, some clinical evidence suggests that constipation and intestinal obstruction, the latter being seen in the disease of cystic fibrosis, can result when secretion is abnormally low. The majority of the intestinal secretory flow of fluid into the lumen is driven by the active secretion of chloride ions via the mechanism diagrammed in Figure 29-18. Some segments of the intestine may engage in additional secretory mechanisms, such as secretion of bicarbonate ions via the mechanisms shown in Figure 29-19. Presumably, this local bicarbonate protects the epithelium, particularly in the most proximal portions of the duodenum immediately downstream from the pylorus, from damage caused by acid and pepsin.
|
| page 529 |  | | page 530 |
| Figure 29-18 Mechanism of Cl- secretion in the small and large intestines. |
| MOTOR PATTERNS OF THE SMALL INTESTINE
|
| Based on the discussions in preceding chapters of this section, it should be possible to predict that the smooth muscle layers of the small intestine act to mix chyme with the various digestive secretions and to move it along the length of the intestine so that its nutrients (along with water and electrolytes) can be absorbed. Motor patterns of the small intestine during the postprandial period are directed predominantly toward mixing and consist largely of segmenting and retropulsive contractions that retard the meal while digestion is still ongoing. Segmentation is a stereotypical pattern of rhythmic contractions that is displayed in Figure 29-20 and presumably reflects programmed activity of the enteric nervous system superimposed on the basic electrical rhythm. Hormonal mediators of the fed pattern of motility are poorly defined, although CCK probably contributes. CCK also plays important roles in slowing gastric emptying when the meal is in the small intestine, as described at the beginning of this chapter. This makes sense as a mechanism to match nutrient delivery to the available capacity to digest and absorb the components of the meal.
|
|
| Figure 29-19 Mechanisms of bicarbonate secretion in the duodenum. CA, carbonic anhydrase. |
| page 530 |  | | page 531 |
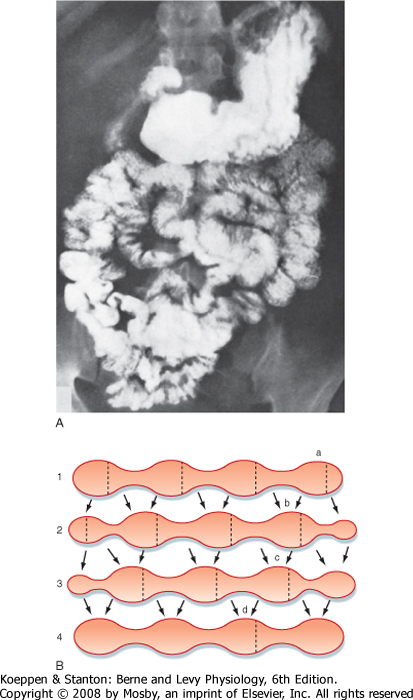
| Figure 29-20 A, Radiographic view showing the stomach and small intestine filled with barium contrast medium in a normal individual. Note the segmentation of the intestine. B, Sequence of segmental contractions in the small intestine. Lines 1 to 4 represent sequential time points. The dotted lines indicate where contractions will occur next; the arrows depict the direction of movement of the intestinal contents. (A, From Gardener EM et al: Anatomy: A Regional Study of Human Structure, 4th ed. Philadelphia, Saunders, 1975; B, redrawn from Cannon WB: Am J Physiol 6:251, 1902.) |
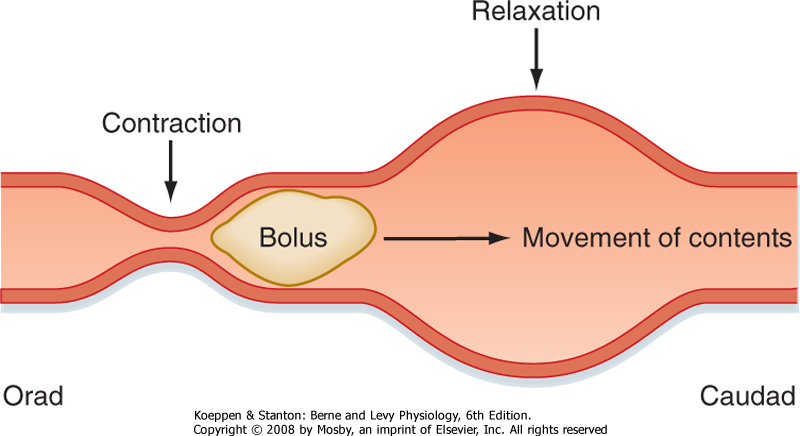
| Figure 29-21 Peristaltic motility in the intestine propels the intestinal contents along the length of the small intestine. |
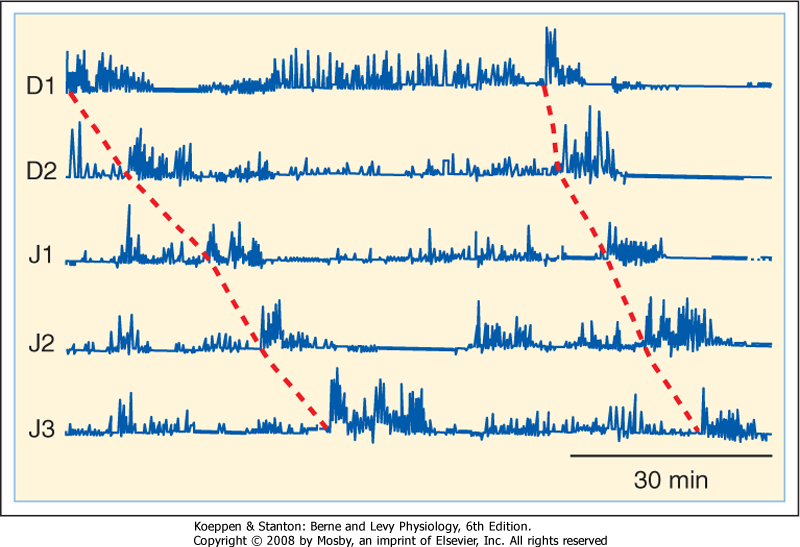
| Figure 29-22 Migrating motor complexes in the duodenum and jejunum as recorded from a fasting human subject by manometry. D1, D2, J1, J2, and J3 indicate sequential recording points along the length of the duodenum and jejunum. The intense contractions (phase III) propagate aborally. (Redrawn from Soffer EE et al: Am J Gastroenterol 93:1318, 1998.) |
| After the meal has been digested and absorbed, it is desirable to clear any undigested residues from the
lumen to prepare the intestine for the next meal. Such clearance is effected by peristalsis (Fig. 29-21), a coordinated sequence of contraction occurring above the intestinal contents and relaxation below that permits the contents to be conveyed over considerable distances. Peristalsis reflects the action of acetylcholine and substance P released orad to a site of intestinal distention, which serves to contract the circular muscle, as well as the inhibitory effects of VIP and nitric oxide on the caudad side. Like segmentation, peristalsis originates when action potentials generated by intrinsic innervation are superimposed on sites of cellular depolarization dictated by the basic electrical rhythm. The peristaltic motor patterns that occur during fasting, moreover, are organized into a sequence of phases known as the migrating motor complex (Fig. 29-22). Phase I of the MMC is characterized by relative quiescence, whereas small disorganized contractions begin to occur during phase II. During phase III, which lasts about 10 minutes, large contractions that propagate along the length of the intestine are stimulated by the hormone motilin and sweep any remaining gastric and intestinal contents out into the colon. The pylorus and ileocecal valve open fully during this phase, so even large undigested items can eventually pass from the body. Motility of the intestine then reverts to phase I of the MMC, with the entire cycle taking about 90 minutes in adults
unless a meal is ingested, in which case the MMC is suspended. After the meal, motilin levels fall (although the mechanisms are unclear), and the MMC cannot be resumed until they rise again.
|
| page 531 |  | | page 532 |
- On leaving the stomach, the meal enters the small intestine, which consists (sequentially) of the duodenum, jejunum, and ileum. The principal function of the small intestine is to digest and absorb the nutrients contained in the meal.
- The presence of chyme in the duodenum retards additional gastric emptying, thus helping match nutrient delivery to the ability of the small intestine to digest and absorb such substances.
- Digestion and absorption in the small intestine are aided by two digestive juices derived from the pancreas (pancreatic juice) and liver (bile). These secretions are triggered by hormonal and neural
signals activated by the presence of the meal in the small intestine.
- Pancreatic secretions arise from the acini and contain various proteins capable of digesting the meal or acting as important cofactors. The secretion is diluted and alkalinized as it passes through the pancreatic ducts.
- Bile is produced by the liver and stored in the gallbladder until needed in the postprandial period. Bile acids, important components of bile, are biological detergents that solubilize the products of lipid digestion.
- Carbohydrates and proteins, water-soluble macromolecules, are digested and absorbed by broadly analogous mechanisms. Lipids, the third macronutrient, require special mechanisms to transfer the products of lipolysis to the epithelial surface, where they can be absorbed.
- The small intestine transfers large volumes of fluid into and out of the lumen on a daily basis to facilitate the digestion and absorption of nutrients, driven by the active transport of ions and other electrolytes.
- The motor patterns of the small intestine vary depending on whether a meal has been ingested. Immediately after a meal, motility is directed to retaining the meal in the small intestine, mixing it with digestive juices, and providing sufficient time for absorption of nutrients. During fasting, a "housekeeper" complex of intense contractions (the migrating motor complex) sweeps periodically along the length of the stomach and small intestine to clear them of undigested residues.
|
 |
|