| 20 Structure and Function of the Respiratory System
|
| The primary function of the lung is gas exchange, which consists of movement of O2 into the body and removal of CO2. The lung also plays a role in host defense by functioning as a primary barrier between the outside world and the inside of the body. Finally, the lung is a metabolic organ that synthesizes and metabolizes numerous compounds. This chapter provides an overview of lung anatomy (i.e., upper and lower airways, muscles, innervation), growth and development of the normal and aging lung, and the fluids lining various anatomic sites, with special emphasis on unique features relative to the lung. Metabolic features of the lung are discussed in Chapter 25.
|
| The lungs are contained in a space with a volume of approximately 4 L, but they have a surface area for gas exchange that is the size of a tennis court (∼85 m2). This large surface area is composed of myriads of independently functioning respiratory units. Unlike the heart but similar to the kidneys, the lungs demonstrate functional unity; that is, each unit is structurally identical and functions just like every other unit. Because the divisions of the lung and the sites of disease are designated by their anatomic locations (right upper lobe, left lower lobe, etc.), it is essential to understand pulmonary anatomy in order to clinically relate respiratory physiology and pathophysiology. In adults, the lung weighs approximately 1 kg, with lung tissue accounting for 60% of the weight and blood the remainder. Alveolar spaces are responsible for most of the lung's volume; these spaces are divided by tissue known collectively as the interstitium. The interstitium is composed primarily of lung collagen fibers and is a potential space for fluid and cells to accumulate.
|
| Upper Airways-Nose, Sinuses, Larynx
|
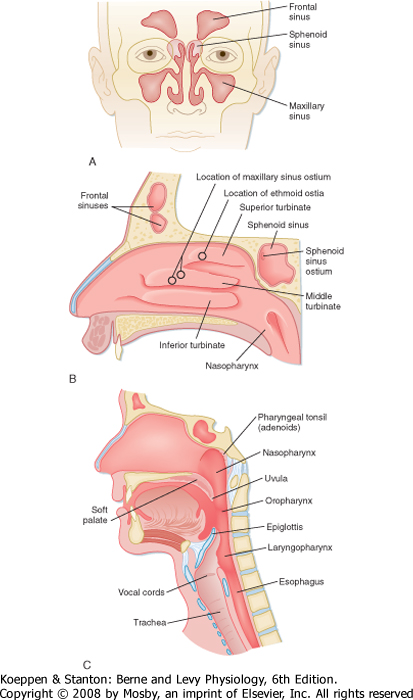
| The respiratory system begins at the nose and ends in the most distal alveolus. Thus, the nasal cavity, the posterior pharynx, the glottis and vocal cords, the trachea, and all divisions of the tracheobronchial tree are included in the respiratory system. The upper airway consists of all structures from the nose to the vocal cords, including sinuses and the larynx, whereas the lower airway consists of the trachea, airways, and alveoli. The major function of the upper airways is to "condition" inspired air so that by the time it reaches the trachea, it is at body temperature and fully humidified. The nose also functions to filter, entrap, and clear particles larger than 10 μm in size. Finally, the nose provides the sense of smell. Neuronal endings in the roof of the nose above the superior turbinate carry impulses through the cribriform plate to the olfactory bulb. The volume of the nose in an adult is approximately 20 mL, but its surface area is greatly increased by the nasal turbinates, which are a series of three continuous ribbons of tissue that protrude into the nasal cavity (Fig. 20-1). In humans, the volume of air entering the nares each day is on the order of 10,000 to 15,000 L. Resistance to airflow in the nose during quiet breathing accounts for approximately 50% of the total resistance of the respiratory system, which is about 8 cm H2O/L/sec. Nasal resistance increases with viral infections and with increased airflow, such as during exercise. When nasal resistance becomes too high, mouth breathing begins.
|
| The interior of the nose is lined by respiratory epithelium interspersed with surface secretory cells. These secretory cells produce important immunoglobulins, inflammatory mediators, and interferons, which are the first line in host defense. The paranasal sinuses, including the frontal sinuses, the maxillary sinus, the sphenoid sinus, and the ethmoid sinus, are lined by ciliated epithelium, and they nearly surround the nasal passages. The cilia facilitate the flow of mucus from the upper airways and clear the main nasal passages approximately every 15 minutes. The sinuses have two major functions-they lighten the skull, which makes upright posture easier, and they offer resonance to the voice. They may also protect the brain during frontal trauma. The fluid covering their surface is continually being propelled into the nose. In some sinuses (e.g., the maxillary sinus), the opening (ostium) is at the upper edge, which makes them particularly susceptible to retention of mucus. The ostia are readily obstructed in the presence of nasal edema, and retention of secretions and secondary infection (sinusitis) can result.
|
| page 417 |  | | page 418 |
| Figure 20-1 Anterior and lateral view drawings of the head and neck illustrating upper airway anatomy. A, Anterior view of the paranasal sinuses. B, Lateral view of the nasal passage structures demonstrating the superior, middle, and inferior turbinates and sinus ostia. C, Lateral midsagittal section of the head and neck showing the three divisions of the pharynx and surrounding upper airway structures. |
| The major structures of the larynx include the epiglottis, arytenoids, and vocal cords (Fig. 20-1). With some infections, these structures can become edematous (swollen) and contribute significantly to airflow
resistance. The epiglottis and arytenoids "hood" or cover the vocal cords during swallowing. Thus, under normal circumstances, the epiglottis and arytenoids function to prevent aspiration of food and liquid into the lower respiratory tract. The act of swallowing food after mastication (chewing) usually occurs within 2 seconds, and it is closely synchronized with muscle reflexes that coordinate opening and closing of the airway. Hence, air is allowed to enter the lower airways and food and liquids are kept out. Patients with some neuromuscular diseases have altered muscle reflexes and can lose this coordinated swallowing mechanism. Thus, they may become susceptible to aspiration of food and liquid, which poses a risk for pneumonia.
|
| Lower Airways-Trachea, Bronchi, Bronchioles, Respiratory Unit
|
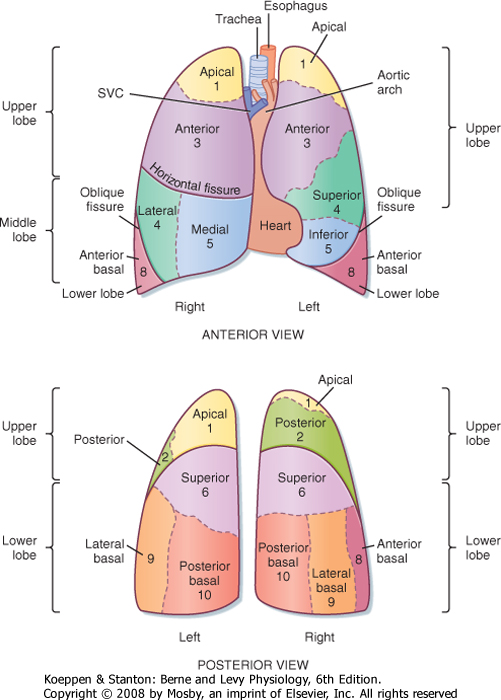
| The right lung, located in the right hemithorax, is divided into three lobes (upper, middle, and lower) by two interlobular fissures (oblique, horizontal), whereas the left lung, located in the left hemithorax, is divided into two lobes (upper, including the lingula, and lower) by an oblique fissure (Fig. 20-2). Both the right and left lungs are covered by a thin membrane called the visceral pleura and are encased by another membrane called the parietal pleura. The interface of these two pleuras allows for smooth gliding of the lung as it expands in the chest and produces a potential space. Air can enter between the visceral and parietal pleuras because of either trauma, surgery, or rupture of a group of alveoli creating a pneumothorax. Fluid can also enter this space and create a pleural effusion or, in the case of severe infection, empyema.
|
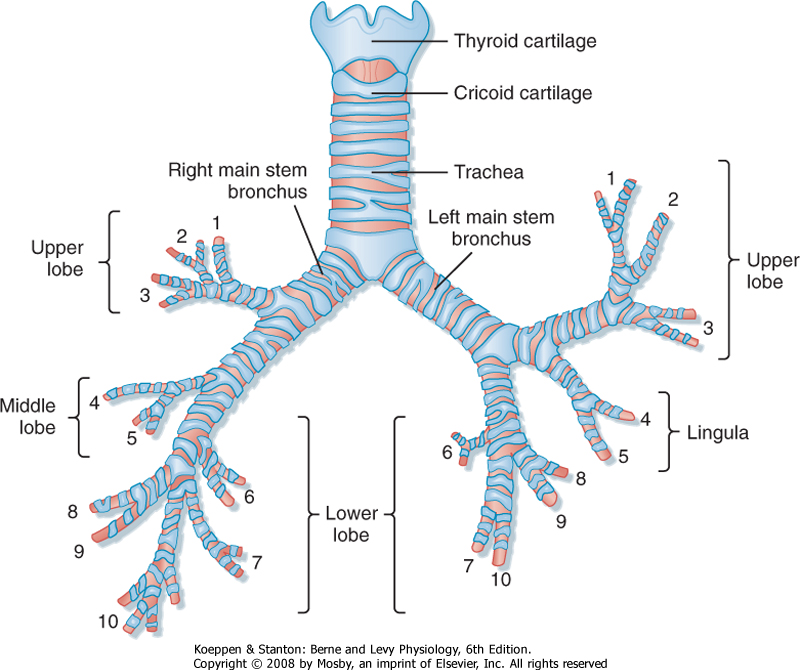
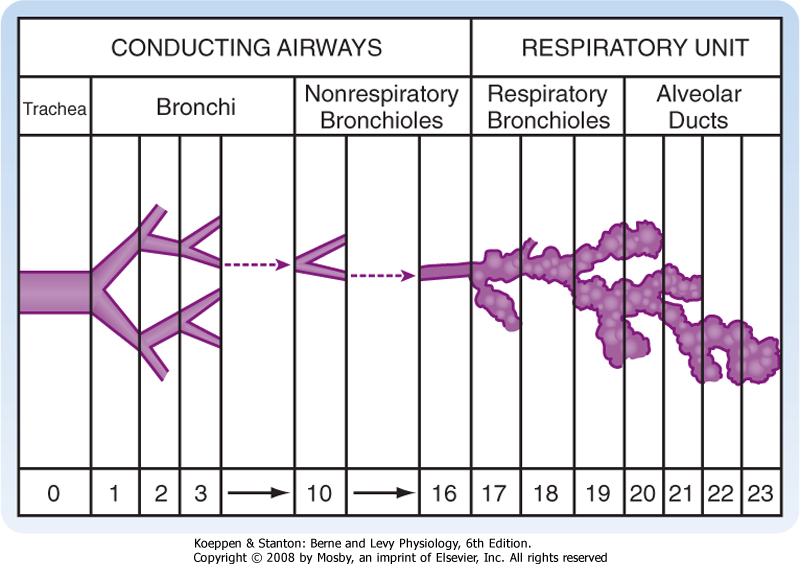
| The trachea bifurcates (branches) into two main stem bronchi (Fig. 20-3). These main stem bronchi then divide (like the branches of a tree) into lobar bronchi (one for each lobe), which in turn divide into segmental bronchi (Figs. 20-3 and 20-4) and into smaller and smaller branches (bronchioles) until reaching the alveolus (Fig. 20-5). The region of the lung supplied by a segmental bronchus is the functional anatomic unit of the lung. Bronchi and bronchioles differ not only in size but also by the presence of cartilage, the type of epithelium, and their blood supply (Table 20-1). The airways continue to divide in a dichotomous or asymmetric branching pattern until they form terminal bronchioles that are distinguished by being the smallest airways without alveoli. Each branching of the respiratory bronchioles results in decreased diameter; however, the total surface area for that generation increases in size and number until the respiratory bronchiole terminates in an opening to a group of alveoli (Fig. 20-5).
|
| page 418 |  | | page 419 |
| Figure 20-2 Topography of the lung demonstrating the lobes, segments, and fissures. The fissures (or chasms) demarcate the lobes in each lung. Numbers refer to specific bronchopulmonary segments, as presented in Figure 20-3. SVC, superior vena cava. |
| Figure 20-3 Bronchopulmonary segments, anterior view: 1, apical; 2, posterior; 3, anterior; 4, lateral (superior); 5, medial (inferior); 6, superior; 7, medial basal; 8, anterior basal; 9, lateral basal; 10, posterior basal. The numbers are the same as in Figure 20-2. |
| page 419 |  | | page 420 |
|
Table 20-1.
Anatomic Characteristics of Bronchi and Bronchioles |
| | Cartilage | Diameter (mm) | Epithelium | Blood Supply | Alveoli | Volume (mL) |
| Bronchi | Yes | >1 | Pseudostratified Columnar | Bronchial | No | - |
| Terminal bronchioles | No | <1 | Cuboidal | Bronchial | No | >150 |
| Respiratory bronchioles | No | <1 | Cuboidal | Pulmonary | Yes | 2500 |
| Figure 20-4 Conducting airways and alveolar units of the lung. The relative size of the alveolar unit is greatly enlarged. Numbers at the bottom indicate the approximate number of generations from trachea to alveoli, which may vary from as few as 10 to as many as 27. (From Weibel ER: Morphometry of the Human Lung. Heidelberg, Germany, Springer-Verlag, 1963.) |
| The conducting airways are involved in several major pulmonary diseases collectively referred to as chronic obstructive pulmonary disease (COPD), including asthma, bronchiolitis, chronic bronchitis, and cystic fibrosis. Obstruction of airflow through the airways is commonly caused by increased mucus, airway inflammation, and smooth muscle constriction. Asthma involves both large and small airways and is characterized by inflammation, predominantly mediated by lymphocytes and eosinophils, in the airways and reversible airway smooth muscle constriction (bronchospasm). Bronchiolitis is a disease of the small airways. It usually occurs in young infants and is caused by viruses, most commonly respiratory syncytial virus. Chronic bronchitis, a disease of smokers, is associated with a marked increase in mucus-secreting cells in the airways and an increase in mucus production. Cystic fibrosis is a genetically inherited disease that adversely affects chloride channels in exocrine glands. In the lung this results in obstruction via abnormal mucus accumulation and leads to recurrent pulmonary infections. |
 |
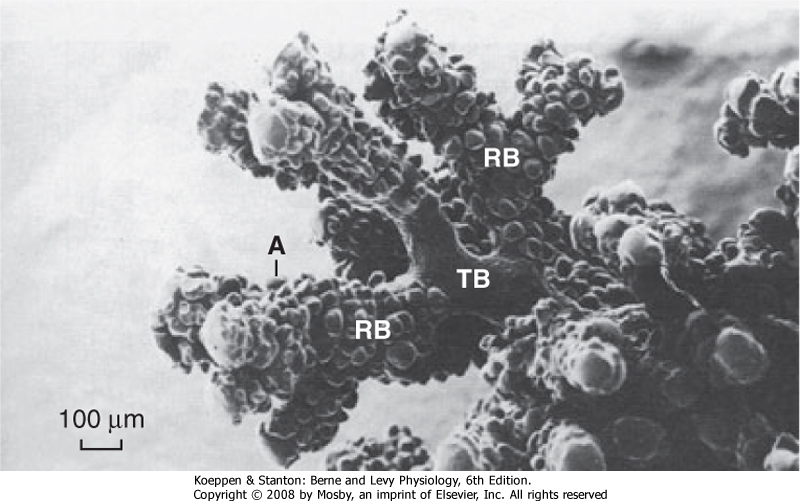
|
| Figure 20-5 The airway from the terminal bronchiole to the alveolus. A, alveolus; RB, respiratory bronchiole; TB, terminal bronchiole. Note the absence of alveoli in the terminal bronchiole. |
| The region of the lung supplied by a segmental bronchus is called a bronchopulmonary segment and is the functional anatomic unit of the lung. Because of its structure, segments of the lung that have become irreversibly diseased can easily be surgically removed. The basic physiological unit of the lung is the respiratory or gas-exchanging unit (respiratory unit), which consists of the respiratory bronchioles, the alveolar ducts, and the alveoli (Figs. 20-4 and 20-5). Bronchi
that contain cartilage and nonrespiratory bronchioles (i.e., lacking alveoli) in which cartilage is absent serve to move gas from the airways to the alveoli and are referred to as the conducting airways. This area of the lung is greater than 150 mL in volume (or ≈30% of a normal breath), does not participate in gas exchange, and forms the anatomic dead space. The respiratory
bronchioles with alveoli and the area from the terminal or nonrespiratory bronchioles to the alveoli are where all gas exchange occurs. This region is only approximately 5 mm long, but it is the single largest volume of the lung at approximately 2500 mL and has a surface area of 70 m2 when the lung and chest wall are at the resting volume (Table 20-1).
|
| page 420 |  | | page 421 |
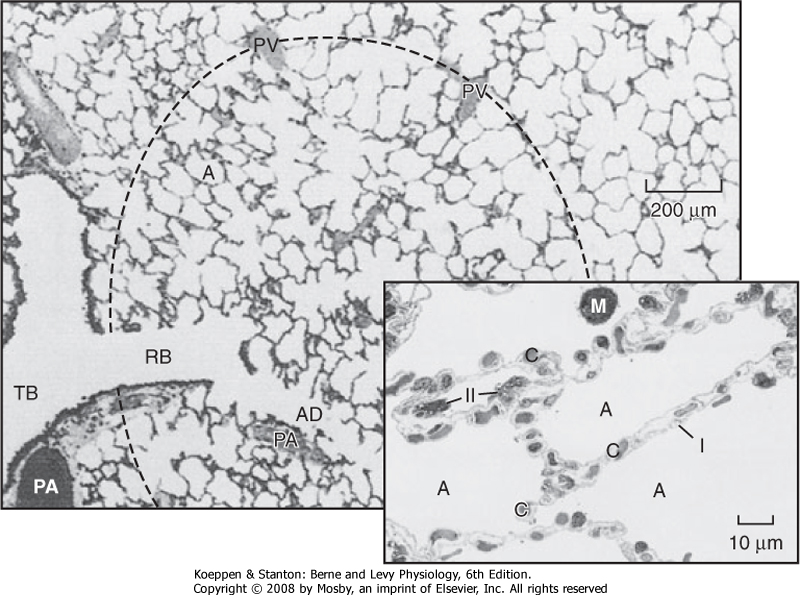
| Figure 20-6 Alveoli. The terminal respiratory unit consists of the alveoli (A) and the alveolar ducts (AD) arising from a respiratory bronchiole (RB). Each unit is roughly spherical, as suggested by the dashed outline. Pulmonary venous vessels (PV) are peripherally located. TB identifies a terminal bronchiole. PA identifies a pulmonary artery. Figure insert, Type I and II alveolar cells. A large fraction of the alveolar wall consists of capillaries (C) and their contents. The alveolar walls are folded and the alveoli (A) are collapsed because this section is from a human lung surgical specimen that was excised and immediately immersed in fixative. This procedure also accounts for the red blood cells in the alveolar air spaces. M, alveolar macrophage; I, type I cell; II, type II cell. |
| The alveoli are polygonal in shape and about 250 μm in diameter. An adult has around 5 × 108 alveoli (Fig. 20-6), which are composed of type I and type II epithelial cells. Under normal conditions type I and type II cells exist in a 1:1 ratio. The type I cell occupies 96% to 98% of the surface area of the alveolus, and it is the primary site for gas exchange. The thin cytoplasm
of type I cells is ideal for optimal gas diffusion. In addition, the basement membrane of type I cells and the capillary endothelium are fused, which minimizes the distance for gas diffusion and thereby facilitates gas exchange. The type II epithelial cell is small and cuboidal and is usually found in the "corners" of the alveolus, where it occupies 2% to 4% of its surface area. Type II cells synthesize pulmonary surfactant, which reduces surface tension in the alveolar fluid and is responsible for regeneration of the alveolar structure subsequent to injury.
|
| Gas exchange occurs in the alveoli through a dense meshlike network of capillaries and alveoli called the alveolar-capillary network. The barrier between gas in the alveoli and the red blood cell is only 1 to 2 μm in thickness and consists of type I alveolar epithelial cells, capillary endothelial cells, and their respective basement membranes. O2 and CO2 passively diffuse across this barrier into plasma and red blood cells. Red blood cells pass through the network in less than 1 second, which is sufficient time for CO2 and O2 gas exchange.
|
| In response to injury and type I cell death, the type II cell replicates and differentiates into type I cells to restore normal alveolar architecture. This repair process is an example of phylogeny recapitulating ontogeny because during embryonic development the epithelium of the alveolus is entirely composed of type II cells and only very late in gestation do type II cells differentiate into type I cells and form the "normal" alveolar epithelium for optimal gas exchange.
|
| The lung interstitial space or interstitium is composed of connective tissue, smooth muscle, lymphatics, capillaries, and a variety of other cells. Under normal conditions the interstitial space is very small and at times cannot be discerned by light microscopy, especially in alveolar compartments. However, in pathological conditions it can become enlarged with the influx of inflammatory cells and edema fluid, which can interfere with gas exchange in the alveoli.
|
| Fibroblasts are prominent cells in the interstitium of the lung. They synthesize and secrete collagen and elastin, which are the extracellular proteins that play a major role in matrix formation and in the physiology of the lung. Collagen is the major structural component of the lung that limits lung distensibility. Elastin is the major contributor to elastic recoil of the lung. Cartilage is a tough, resilient connective tissue that supports the conducting airways of the lung and encircles about 80% of the trachea. The amount of cartilage decreases down the respiratory system and disappears at the level of the bronchioles. In addition to cartilage, the airway epithelium rests on spiral bands of smooth muscle, which can dilate or constrict in response to chemical, irritant, or mechanical stimulation. Kultschitzky cells, neuroendocrine cells, are found in clumps throughout the tracheobronchial tree and secrete biogenic amines, including dopamine and 5-hydroxytryptamine (serotonin). These cells are more numerous in a fetus than in an adult, and they appear to be the cells of origin for a rare bronchial tumor called bronchial carcinoid.
|
| BLOOD SUPPLY TO THE LUNG-PULMONARY AND BRONCHIAL CIRCULATIONS
|
| page 421 |  | | page 422 |
| The lung has two separate blood supplies. The pulmonary circulation brings deoxygenated blood from the right ventricle to the gas-exchanging units for removal of CO2 and oxygenation before blood is returned to the left atrium for distribution to the rest of the body. The
bronchial circulation arises from the aorta and provides nourishment to the lung parenchyma. The circulation to the lung is unique in its duality and ability to accommodate large volumes of blood at low pressure.
|
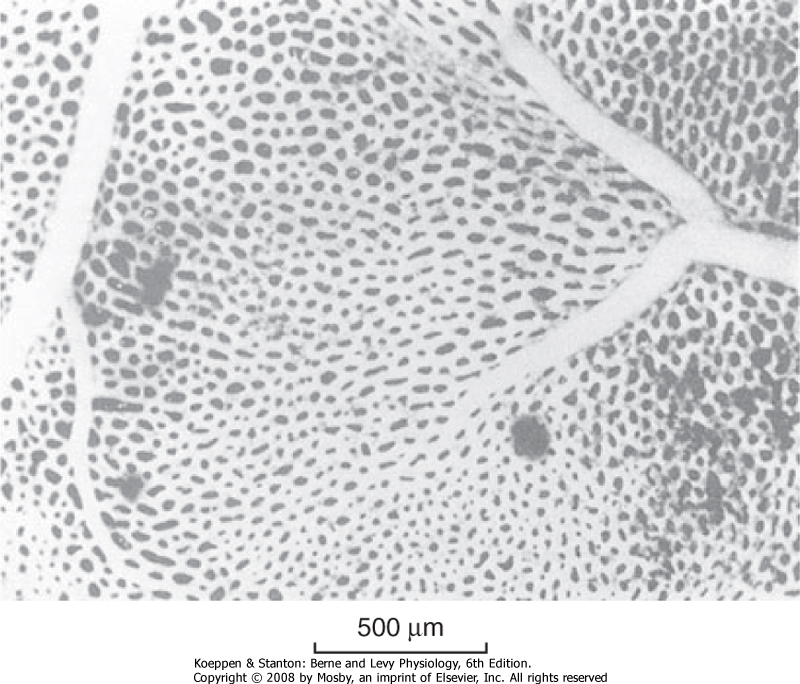
| The pulmonary capillary bed is the largest vascular bed in the body. It covers a surface area of 70 to 80 m2, which is nearly as large as the alveolar surface area. The network of capillaries is so dense that it might be considered to be a sheet of blood interrupted by small vertical supporting posts (Fig. 20-7). The capillary volume in the lung at rest is approximately 70 mL. During exercise, this volume increases and approaches 200 mL. This increase occurs, in part, through the recruitment of closed or compressed capillary segments as increased cardiac output raises pulmonary vascular pressure. In addition, open capillaries can enlarge as their internal pressure rises. This occurs when the lungs fill with blood, as it does in left heart failure, which is associated with elevated left atrial pressure. The pulmonary veins return blood to the left atrium through supernumerary conventional branches. Because of their larger numbers and thinner walls, the pulmonary veins provide a large reservoir for blood, and they can either increase or decrease their capacitance to provide constant left ventricular output in the face of variable pulmonary arterial flow. Pulmonary arteries and veins with diameters larger than 50 μm contain smooth muscle. These vessels actively regulate their diameter and thus alter resistance to blood flow.
|
| Figure 20-7 View of an alveolar wall showing the dense network of capillaries. A small artery and vein can also be seen. The individual capillary segments are so short that the blood forms an almost continuous sheet. (From Maloney JE, Castle BL: Respir Physiol 7:150, 1969.) |
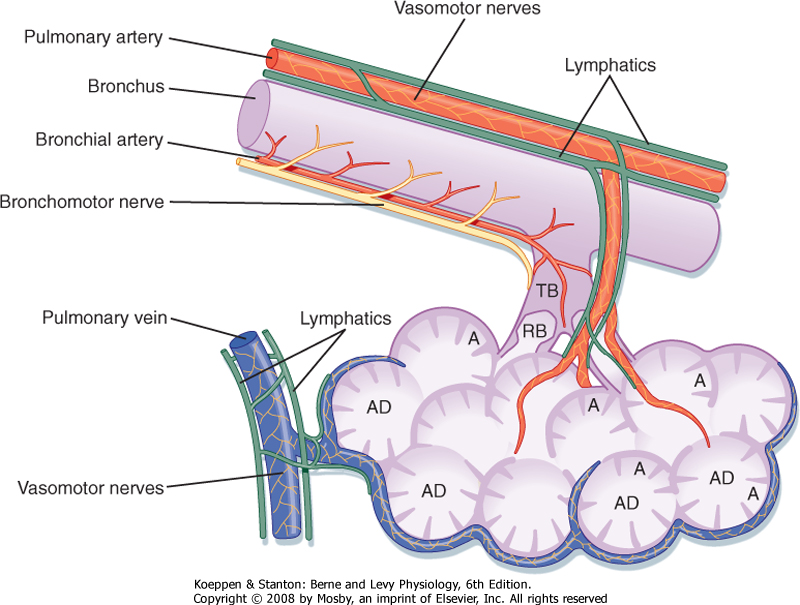
| Figure 20-8 Anatomic relationship between the pulmonary artery, the bronchial artery, the airways, and the lymphatics. A, alveoli; AD, alveolar ducts; RB, respiratory bronchioles; TB, terminal bronchioles. |
| page 422 |  | | page 423 |
| The bronchial arteries, usually three in number, provide a source of oxygenated, systemic blood to the lungs. These arteries accompany the bronchial tree and divide with it (Fig. 20-8). They nourish the walls of the bronchi, bronchioles, blood vessels, and nerves, and they perfuse the lymph nodes and most of the visceral pleura. Approximately a third of the blood returns to the right atrium through the bronchial veins, whereas the remainder drains into the left atrium via pulmonary veins. In the presence of diseases such as cystic fibrosis, the bronchial arteries,
which normally receive only 1% to 2% of cardiac output, increase in size (hypertrophy) and receive as much as 10% to 20% of cardiac output. The erosion of inflamed tissue into these vessels secondary to bacterial infection is responsible for the hemoptysis (coughing up blood) that occurs in this disease.
|
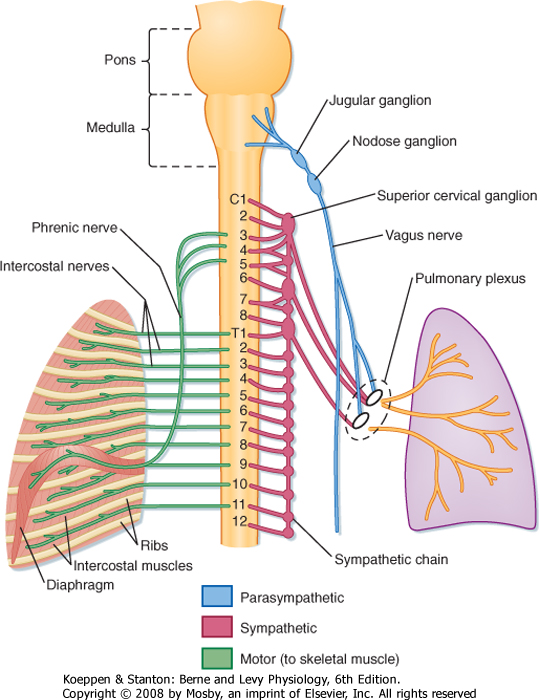
| Figure 20-9 Innervation of the lungs. The autonomic innervation (motor and sensory) of the lung and the somatic (motor) nerve supply to the intercostal muscles and diaphragm are depicted. |
| Breathing is automatic and under control of the central nervous system (CNS). The peripheral nervous system (PNS) includes both sensory and motor components. The PNS conveys and integrates signals from the environment to the CNS. Sensory and motor neurons of the PNS transmit signals from the periphery to the CNS. Somatic motor neurons innervate skeletal muscles, and autonomic neurons innervate smooth muscle, cardiac muscle, and glands. The lung is innervated by the autonomic nervous system of the PNS, which is under CNS control (Fig. 20-9). There are four distinct components of the autonomic nervous system: parasympathetic (constriction), sympathetic (relaxation), nonadrenergic noncholinergic inhibitory (relaxation),
and nonadrenergic noncholinergic stimulatory (constriction).
|
| Stimulation of the parasympathetic system leads to airway constriction, blood vessel dilation, and increased glandular secretion. Stimulation of the sympathetic system causes airway relaxation, blood vessel constriction, and inhibition of glandular secretion (Fig. 20-10). The functional unit of the autonomic nervous system is composed of preganglionic and postganglionic neurons in the CNS and postganglionic neurons in the ganglia of the specific organ. As with most organ systems, the CNS and PNS work in cohort to maintain homeostasis. There is no voluntary motor innervation in the lung, nor are there pain fibers. Pain fibers are found only in the pleura.
|
| page 423 |  | | page 424 |
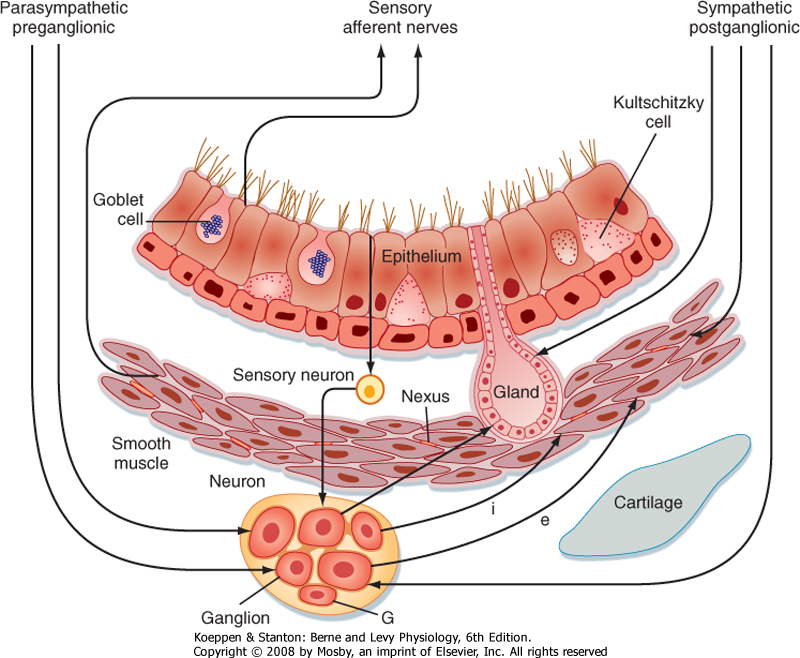
| Figure 20-10 Schematic summary of the innervation of the airways. Parasympathetic, preganglionic fibers descend into the vagus and terminate in the ganglia. The ganglia contain excitatory neurons that are cholinergic and inhibitory neurons that are nonadrenergic. Other neurons with an integrative function are probably also present. Glial cells (G) are present in the ganglia. Postganglionic fibers to the smooth muscle are excitatory (e) or inhibitory (i). |
| The parasympathetic innervation of the lung originates from the medulla in the brainstem (cranial nerve X, the vagus). Preganglionic fibers from the vagal nuclei descend in the vagus nerve to ganglia adjacent to airways and blood vessels in the lung. Postganglionic fibers from the ganglia then complete the network by innervating smooth muscle cells, blood vessels, and bronchial epithelial cells (including goblet cells and submucosal glands). The anatomic locations of
the parasympathetic nervous system enhance specific organ responses without influencing other organs. Both preganglionic and postganglionic fibers contain excitatory (cholinergic) and inhibitory (nonadrenergic) motor neurons. Acetylcholine and substance P are neurotransmitters of excitatory motor neurons; dynorphin and vasoactive intestinal peptide are neurotransmitters of inhibitory motor neurons. Parasympathetic stimulation through the vagus nerve is responsible for the slightly constricted smooth muscle tone in the normal resting lung. Parasympathetic fibers also innervate the bronchial glands, and these fibers, when stimulated, increase the synthesis of mucus glycoprotein, which raises the viscosity of mucus. Parasympathetic innervation is greater in the larger airways, and it diminishes toward the smaller conducting airways in the periphery.
|
| Whereas the response of the parasympathetic nervous system is very specific and local, the response of the sympathetic nervous system tends to be more general. Mucous glands and blood vessels are heavily innervated by the sympathetic nervous system; however, smooth muscles are not. Neurotransmitters of the adrenergic nerves include norepinephrine and dopamine, although dopamine has no influence on the lung. Stimulation of the sympathetic nerves in mucous glands increases water secretion. This upsets the balanced response of increased water and increased viscosity between the sympathetic and parasympathetic pathways. Adrenergic fibers, though present in some animal species, are absent in humans. In addition to the sympathetic and parasympathetic systems, afferent nerve endings are present in the epithelium and in smooth muscle cells.
|
| Central Control of Respiration
|
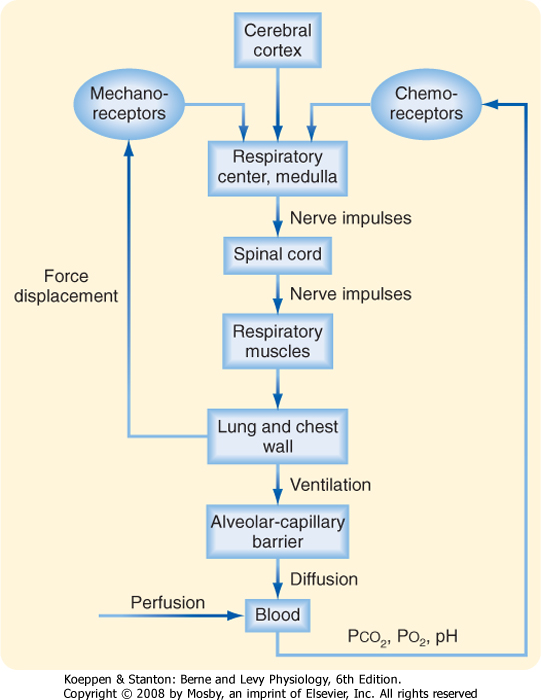
| Breathing is an automatic, rhythmic, and centrally regulated process with voluntary control. The CNS and, in particular, the brainstem function as the main control center for respiration (Fig. 20-11). Regulation of respiration requires (1) generation and maintenance of a respiratory rhythm; (2) modulation of this rhythm by sensory feedback loops and reflexes that allow adaptation to various conditions while minimizing energy costs; and (3) recruitment of respiratory muscles that can contract appropriately for gas exchange.
|
| page 424 |  | | page 425 |
| Figure 20-11 Block diagram of the respiratory control system. |
| The central pattern generator (CPG) is composed of several groups of cells in the brainstem that have the property of a pacemaker. The CPG integrates peripheral input from stretch receptors in the lung and O2 receptors in the carotid body with central input from the hypothalamus and amygdala. This input may be excitatory or inhibitory. In addition, because phrenic nerve output is absent between inspiratory efforts, an inspiratory on-off switch appears to operate
in the system, and this switch inhibits the CPG during exhalation. Control of respiration is described in greater detail in Chapter 24.
|
| MUSCLES OF RESPIRATION-DIAPHRAGM, EXTERNAL INTERCOSTALS, SCALENE
|
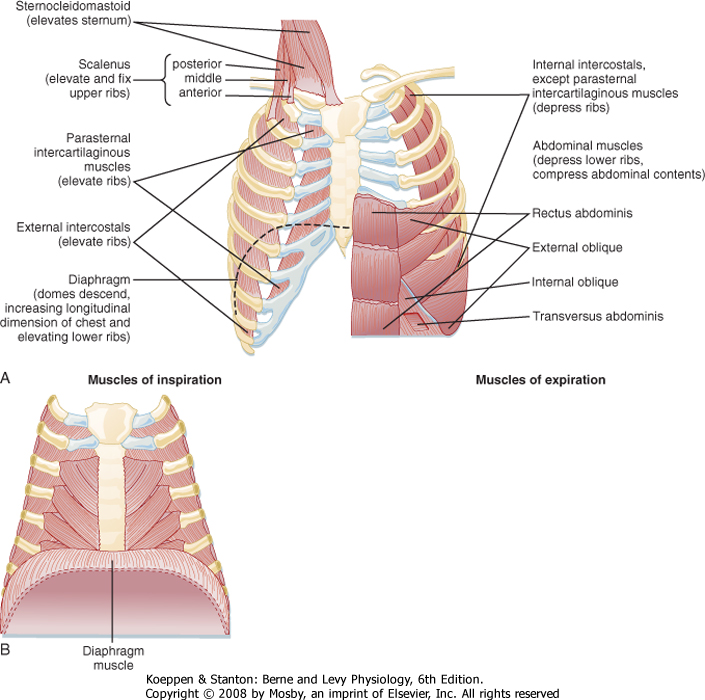
| The major muscles of respiration include the diaphragm, the external intercostals, and the scalene, which are all skeletal muscles. Skeletal muscles provide the driving force for ventilation; the force of contraction increases when they are stretched and decreases when they shorten. The force of contraction of respiratory muscles increases at larger lung volumes.
|
| The process of respiration or gas exchange begins with the act of inspiration, which is initiated by contraction of the diaphragm. On contraction, the diaphragm protrudes into the abdominal cavity and moves the abdomen outward to create negative pressure inside the chest. The opening of the glottis in the upper airway creates a portal that connects the outside world to the respiratory system. Because gases flow from higher to lower pressure, air moves into the lungs from the outside, much like the way a vacuum cleaner sucks air into the canister. Lung volume increases at inspiration and oxygen (O2) is taken into the lung, whereas during exhalation the diaphragm relaxes, pressure inside the chest increases, and carbon dioxide (CO2) and other gases flow passively out of the lungs.
|
| The diaphragm is the major muscle of respiration and it divides the thoracic cavity from the abdominal cavity (Fig. 20-12). Contraction of the diaphragm forces the abdominal contents downward and forward. This increases the vertical dimension of the chest cavity and creates a pressure difference between the thorax and abdomen. In adults, the diaphragm can generate airway pressures of up to 150 to 200 cm H2O during maximal inspiratory effort. During quiet breathing (tidal breathing), the diaphragm moves approximately 1 cm; however, during deep-breathing maneuvers (vital capacity), the diaphragm can move as much as 10 cm. The diaphragm is innervated by the right and left phrenic nerves, which have their origins at the third to fifth cervical segments of the spinal cord (C3 to C5).
|
| The other important muscles of inspiration are the external intercostal muscles, which pull the ribs upward and forward during inspiration (Fig. 20-10). This causes an increase in both the lateral and anteroposterior diameter of the thorax. Innervation of the external intercostal muscles originates from intercostal nerves that arise from the same level of the spinal cord. Paralysis of these muscles has no significant effect on respiration because respiration is primarily dependent on the diaphragm. This is why individuals with high spinal cord injuries can breathe on their own. It is only when the injury is above C3 that individuals are completely dependent on a ventilator.
|
| Accessory muscles of inspiration (the scalene muscles, which elevate the sternocleidomastoid; the alae nasi, which cause nasal flaring; and small muscles in the neck and head) do not contract during normal breathing. However, they do contract vigorously during exercise, and when airway obstruction is significant, they actively pull up on the rib cage. During normal breathing they anchor the sternum and upper ribs. Because the upper airway must remain patent during inspiration, the pharyngeal wall muscles (genioglossus and arytenoid) are also considered to be muscles of inspiration. All of the rib cage muscles are voluntary muscles that are supplied by intercostal arteries and veins and innervated by motor and sensory intercostal nerves.
|
| page 425 |  | | page 426 |
| Figure 20-12 The major respiratory muscles. A, The inspiratory muscles are on the left side, and the expiratory muscles are on the right side. B, The diaphragm muscle in relation to the rib cage. (From Garrity ER, Sharp JT. In Pulmonary and Critical Care Update, vol 2. Park Ridge, IL, American College of Chest Physicians, 1986.) |
| Exhalation during normal breathing is passive, but it becomes active during exercise and hyperventilation. The most important muscles of exhalation are those of the abdominal wall (rectus abdominis, internal and external oblique, and transversus abdominis) and the internal intercostal muscles, which oppose the external intercostal muscles (i.e., they pull the ribs downward and inward). The inspiratory muscles do the work of breathing. During normal breathing, work is low and the inspiratory muscles have significant reserve. Respiratory muscles can be trained to do more work, but there is a finite limit to the work that they can perform. Respiratory muscle weakness can impair movement of the chest wall, and
respiratory muscle fatigue is a major factor in the development of respiratory failure.
|
| FLUIDS LINE THE LUNG EPITHELIUM AND PLAY IMPORTANT PHYSIOLOGICAL ROLES
|
| The respiratory system is lined with three very different and highly significant fluids: periciliary fluid, mucus, and surfactant. Periciliary fluid and mucus are components of the mucociliary clearance system and line the epithelium of the conducting airways from the trachea to the terminal bronchioles. Together they form the basis for the mucociliary clearance system, which aids in the removal of particulates (e.g., bacteria, viruses, toxins) from the lung and will be discussed further in Chapter 25. Surfactant lines the epithelium of the alveolus and provides an "antistick" function that decreases surface tension in the alveolus. The cells involved in mucociliary clearance and the production of mucus and surfactant are described next.
|
| Cells of the Mucociliary Clearance System
|
| The respiratory tract to the level of the bronchioles is lined by a pseudostratified, ciliated columnar epithelium (Fig. 20-10). These cells maintain the level of periciliary fluid, a 5-μm layer of water and electrolytes in which cilia and the mucociliary transport system function. The depth of the periciliary fluid is maintained by the movement of various ions, mainly chloride secretion and sodium absorption, across the epithelium. Mucus and inhaled particles are removed from the airways by the rhythmic beating of the cilia on the top of the pseudostratified, columnar epithelium. Each epithelial cell contains about 250 cilia.
|
| page 426 |  | | page 427 |
| Because respiratory muscles provide the driving force for ventilation, diseases that affect the mechanical properties of the lung affect the muscles of respiration. For example, in chronic obstructive pulmonary disease (COPD), the work of breathing is increased secondary to airflow obstruction. Exhalation is no longer passive but requires active, expiratory muscle contraction. In addition, total lung capacity (TLC) is increased. The greater TLC forces the diaphragm downward, shortens the muscle fibers, and decreases the radius of curvature. As a result, the function and efficiency of the diaphragm are decreased. Respiratory muscles can fatigue just as other skeletal muscles do when the workload increases. Respiratory muscles can also weaken in patients with neuromuscular diseases (e.g., Guillain-Barré syndrome, myasthenia gravis). In these diseases, sufficient respiratory muscle weakness can impair movement of the chest wall and result in respiratory failure even though the mechanical properties of the lung and chest wall are normal. |
 |
| Cells Regulating Mucus Production
|
| Goblet or surface secretory cells are interspersed among the epithelial cells in a ratio of approximately one goblet cell to five ciliated cells. They produce mucus in the airways and increase in number in response to chronic cigarette smoke (and environmental pollutants) and thus contribute to the increased mucus and airway obstruction observed in smokers.
|
| Submucosal tracheobronchial glands are present wherever there is cartilage in the tracheobronchial tree. These glands empty to the surface epithelium through a ciliated duct, and they are lined by mucus-secreting mucous and serous cells (Fig. 20-10). Submucosal tracheobronchial glands increase in number and size in chronic bronchitis, and they extend down to the level of the bronchioles in pulmonary disease.
|
| In normal individuals, Clara cells are found at the level of the bronchioles, where the goblet cells and submucosal glands have disappeared. Although their function is controversial, they contain granules with nonmucinous material and may have a secretory function. In addition, they may play a role in epithelial regeneration after injury.
|
| Surfactant and Surface Tension
|
| The alveoli are lined with a predominantly lipid-based substance called surfactant that reduces surface tension. Surface tension is a force caused by water molecules at the air-liquid interface that tends to minimize surface area, thereby making it more difficult to inflate the lung. The effect of surface tension on lung inflation is illustrated by comparing the volume-pressure curves of a saline-filled versus an air-filled lung. Higher pressure is required to fully inflate the lung with air than with saline because of the higher surface tension forces in air-filled versus saline-filled lungs.
|
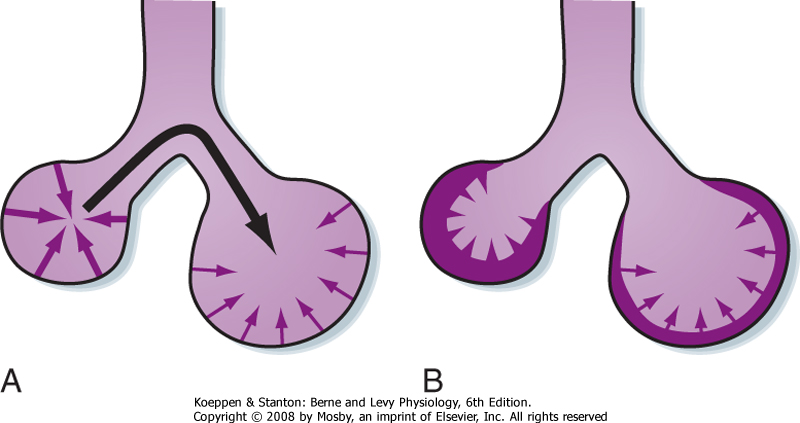
|
| Figure 20-13 Surface forces in a sphere attempt to reduce the area of the surface and generate pressure within the sphere. By Laplace's law, the pressure generated is inversely proportional to the radius of the sphere. A, Surface forces in the smaller sphere generate higher pressure (heavier arrows) than those in the larger sphere (lighter arrows). As a result, air moves from the small sphere (higher pressure) to the larger sphere (lower pressure; black arrow). This causes the small sphere to collapse and the large sphere to become overdistended. B, Surfactant (shaded layer) lowers surface tension and lowers it more in the smaller sphere than in the larger sphere. The net result is that the pressure in the small and larger spheres is similar and the spheres are stabilized. |
Surface tension is a measure of the attractive force of the surface molecules per unit length of material to which they are attached. The units of surface tension are those of a force applied per unit length. For a sphere (such as an alveolus), the relationship between the pressure within the sphere (Ps) and the tension in the wall is described by the law of Laplace:
 where T is the wall tension (dynes/cm) and r is the radius of the sphere.
where T is the wall tension (dynes/cm) and r is the radius of the sphere.
|
| In the absence of surfactant, the surface tension at the air-liquid interface would remain constant and the transmural (transalveolar) pressure needed to keep it at that volume would be greater at lower lung (alveolar) volumes (Fig. 20-13, A). Thus, greater transmural pressure would be required to produce a given increase in alveolar volume at lower lung volumes than at higher lung volumes. Surfactant stabilizes the inflation of alveoli because it allows the surface tension to decrease as the alveoli become larger (Fig. 20-13, B). As a result, the transmural pressure required to keep an alveolus inflated increases as lung volume (and transpulmonary pressure) increases, and it decreases as lung volume decreases. The unique feature of surfactant is that although it decreases the surface tension of alveoli overall, the surfactant can change its surface tension at different lung volumes. Specifically, in the presence of surfactant there is an increase in surface tension at high lung volume and a decrease at low lung volume. The result is that the lung can maintain alveoli at many different volumes. Otherwise, small alveoli would empty into large alveoli.
|
| page 427 |  | | page 428 |
| In addition to surfactant, another mechanism, namely, interdependence, contributes to stability of the alveoli. Alveoli, except for those on the pleural surface, are surrounded by other alveoli. The tendency of one alveolus to collapse is opposed by the traction exerted by the surrounding alveoli. Thus, collapse of a single alveolus stretches and distorts the surrounding alveoli, which in turn are connected to other alveoli. Small openings (pores of Kohn) in the alveolar walls connect adjacent alveoli, whereas the canals of Lambert connect the terminal airways to adjacent alveoli. The pores of Kohn and the canals of Lambert provide collateral ventilation and prevent alveolar collapse (atelectasis).
|
| Composition and Function of Surfactant
|
| Pulmonary surfactant is a complex mixture of phospholipids, neutral lipids, fatty acids, and proteins. Surfactant is 85% to 90% lipids, predominantly phospholipids, and 10% to 15% proteins. The major phospholipid is phosphatidylcholine, approximately 75% of which is present as dipalmitoyl phosphatidylcholine (DPPC). DPPC is the major surface-active component in surfactant, and it decreases surface tension. The second most abundant phospholipid is phosphatidylglycerol (PG), which accounts for 1% to 10% of total surfactant. These lipids are important in formation of the monolayer on the alveolar-air interface, and PG is important in the spreading of surfactant over a large surface area. Surfactant is secreted by type II cells and thus must spread over the entire surface area of the alveolus. This is accomplished with the aid of surfactant components such as PG, which have spreading properties. Cholesterol and cholesterol esters account for the majority of the neutral lipids; their precise functional role is not yet fully understood, but they may aid in stabilizing the lipid structure.
|
| Four specific surfactant proteins (SP-A, SP-B, SP-C, SP-D) make up 2% to 5% of the weight of surfactant. The most studied is SP-A, which is expressed in alveolar type II cells and in Clara cells in the lung. SP-A is involved in the regulation of surfactant turnover, in immune regulation within the lung, and in the formation of tubular myelin. Tubular myelin is the term used to describe a precursor stage of surfactant as it is initially secreted from the type II cell and has not yet spread. Two hydrophobic surfactant-specific proteins are SP-B and SP-C. SP-B may be involved in tubular myelin formation and the surface activity (i.e., surface tension, spreading ability) of surfactant, and it may increase the intermolecular and intramolecular order of the phospholipid bilayer. SP-C may be involved in the spreading ability and surface tension activity of surfactant. The function of SP-D is unknown at this time.
|
| In 1959, Avery and Mead discovered that the lungs of premature infants who died of hyaline membrane disease (HMD) were deficient in surfactant. HMD, also known as infant respiratory distress syndrome (RDS), is characterized by progressive atelectasis and respiratory failure in premature infants. It is a major cause of morbidity and mortality in the neonatal period. The major surfactant deficiency in premature infants is lack of PG. In general, as the level of PG increases in amniotic fluid, the mortality rate decreases. Research in this field has culminated in successful attempts to treat HMD in premature infants with surfactant replacement therapy. Today, surfactant replacement therapy is standard care for premature infants. |
 |
| Secretion of surfactant into the airway occurs via exocytosis of the lamellar body by constitutive and regulated mechanisms. Numerous agents, including β-adrenergic agonists, activators of protein kinase C, leukotrienes, and purinergic agonists, stimulate the exocytosis of surfactant. The major routes of clearance
of pulmonary surfactant within the lung are reuptake by type II cells, absorption into the lymphatics, and clearance by alveolar macrophages. In summary, pulmonary surfactant serves several physiological roles, including (1) reducing the work of breathing by decreasing surface tension forces; (2) preventing collapse and sticking of alveoli on expiration, and (3) stabilizing alveoli, especially those that tend to deflate at low surface tension.
|
| Host defense and removal of lymph fluid from the lung are the major functions of the lymphatic network in the lung. Interstitial fluid enters lymphatic vessels via lymphatic capillaries. The lymphatic fluid drains to larger lymphatic vessels, and it eventually returns to the blood by way of large veins. Changes in tissue pressure and contractions of the lymphatic vessels drive the interstitial fluid into the lymphatic capillaries. The lymphatic capillaries are highly specialized to allow the transfer of fluid from the interstitial spaces into the lymphatic capillaries. Although the lymphatic capillaries are somewhat similar to blood capillaries, they have several distinct features that aid in fluid movement and clearance: (1) there are no tight junctions between endothelial cells, (2) fine filaments anchor the lymph vessels to adjacent connective tissue such that with each muscle contraction the endothelial junctions open to allow fluid movement, and (3) valves enhance lymph flow in one direction. Chapter 25 will provide additional details about the lymphatic system and the immune functions of the lungs.
|
| LUNG DEVELOPMENT, GROWTH, AND AGING
|
| page 428 |  | | page 429 |
| The epithelium of the lung arises as a pouch from the primitive foregut at approximately 22 to 26 days after
fertilization of the ovum. This single lung bud branches into primitive right and left lungs. Over the next 2 to 3 weeks, further branching occurs to create the irregular dichotomous branching pattern. Reid has described "three laws of lung development": (1) the bronchial tree has developed by week 16 of intrauterine life; (2) alveoli develop after birth, the number of alveoli increases until the age of 8 years, and the size of alveoli increases until growth of the chest wall is completed at adulthood; and (3) preacinar vessels (arteries and veins) parallel the development of the airways, whereas intraacinar vessels parallel that of the alveoli. Thus, intrauterine events that occur before 16 weeks of gestation will affect the number of airways. A condition known as congenital diaphragmatic hernia is an example of a congenital lung disease. It occurs at 6 to 8 weeks of gestation and is due to failure of the pleuroperitoneal canal to close and separate the chest and abdominal cavities; it results in a decreased number of alveoli.
|
| Growth of the lungs is similar and relatively proportional to growth in body length and stature. The rate of development is fastest in the neonatal and adolescent (≈11 years of age) periods, with girls maturing earlier than boys. Although the growth rate slows after adolescence, the body and lungs increase in size steadily until adulthood. Improvement in lung function occurs at all stages of growth development; however, once optimal size has been attained in early adulthood (20 to 25 years of age), lung function starts to decline with age. The decrease in lung function with age, estimated at less than 1% per year, appears to begin earlier and proceed faster in individuals who smoke or are exposed to toxic environmental factors. The major physiological insufficiencies caused by aging involve ventilatory capacity and ventilatory responses, especially during exercise, and they result in a mismatch of abnormal ventilation with normal perfusion. In addition, gas diffusion decreases with age, most likely as a result of a decrease in alveolar surface area. Age-related decreases in lung function and altered morphology parallel biochemical observations of increased elastin within the lung, which could explain some of the functional abnormalities.
|
- The lung demonstrates anatomic and physiological unity; that is, each unit (bronchopulmonary segment) is structurally identical and it functions just like every other unit.
- The circulation to the lung is unique in its dual circulation and ability to accommodate large volumes of blood at low pressure. The pulmonary circulation brings deoxygenated blood from the right ventricle to the gas-exchanging units. The bronchial circulation arises from the aorta and provides nourishment to the lung parenchyma.
- Inspiration is the active phase of breathing; the muscles of the chest wall, mainly the diaphragm, contract and move down into the abdomen, thereby resulting in negative pressure inside the chest. Gas then flows from higher to lower pressure.
- The surface tension-reducing and antistick properties of surfactant diminish the work of breathing and help stabilize alveoli.
- The respiratory center is located in the medulla in the brainstem and regulates respiration with input from sensory feedback loops and reflexes in the lung and chest wall and from chemoreceptors that respond to changes in O2 and CO2.
|
 |
|


 where T is the wall tension (dynes/cm) and r is the radius of the sphere.
where T is the wall tension (dynes/cm) and r is the radius of the sphere.