| 5 Generation and Conduction of Action Potentials
|
An action potential is a rapid, all-or-none change in the membrane potential followed by a return to the resting membrane potential.
- Voltage-dependent ion channels in the plasma membrane are the basis for action potentials.
- An action potential is propagated with the same shape and size along the entire length of an axon.
- Action potentials are usually initiated at the initial segment of the axon.
- The action potential is the basis of the signal-carrying ability of nerve cells.
- The patterns of conducted action potentials encode the information conveyed by nerve cells.
|
| This chapter describes how action potentials are generated and conducted. Within this general discussion, the influence of axon geometry, ion channel distribution, and myelin is discussed and explained. The ways in which information is encoded by the frequency and pattern of action potentials in individual cells and in groups of nerve cells are also presented. Finally, because the nervous system provides important information about the external world through specific sensory receptors, the general principles of sensory transduction and coding are reviewed. More detailed information on these sensory mechanisms and systems is provided in other chapters.
|
| Observations of Membrane Potentials
|
| All cells, including neurons, have a resting potential that is typically around -70 mV, as detailed in Chapter 1. One of the signature features of neurons is their ability to change their membrane potential rapidly in response to an appropriate stimulus, and the most significant of these responses is the action potential. Our current knowledge about the ionic mechanisms of action potentials comes from experiments with many species. One of the most studied, however, is the squid because the large diameter (up to 0.5 mm) of the squid giant axon makes it a convenient model for electrophysiological research with intracellular electrodes. When a microelectrode (tip diameter <0.5 μm) is inserted through the plasma membrane of the squid giant axon, a potential difference is observed between the tip of the microelectrode inside the cell and an electrode placed outside the cell. The internal electrode is approximately 70 mV negative with respect to the external electrode. This 70-mV potential difference is the resting membrane potential of the axon. By convention, membrane potentials are expressed as the intracellular potential minus the extracellular potential; therefore, the resting potential of squid giant axons, as well as many mammalian neurons, is about -70 mV. In the absence of perturbing influences, the resting membrane potential remains at -70 mV.
|
| Figure 5-1 illustrates the results of an experiment in which the membrane potential of an axon is perturbed by passing rectangular pulses of depolarizing or hyperpolarizing current across the plasma membrane. The injection of positive charge, which changes the membrane potential from -70 mV to -60 mV, is depolarizing because it makes the cell more positive (i.e., decreases the potential difference across the cell membrane). Conversely, a change in the membrane potential from -70 mV to -80 mV as a result of the injection of negative charge increases the polarization of the membrane; this change in potential is called hyperpolarization. The more current that passes across the plasma membrane, the larger the change in the membrane potential.
|
| page 65 |  | | page 66 |
| Figure 5-1 Responses of an axon to rectangular pulses of hyperpolarizing (a) or depolarizing (b to d) current. The change in transmembrane current and potential as recorded by an intracellular electrode is shown as a function of time. Note that when stimulated to threshold (d), the axon fires an action potential. For clarity, only the rising phase of the action potential is shown. RMP, resting membrane potential. (Redrawn from Blankenship J: Neurophysiology. Philadelphia, Mosby, 2002.) |
| Note that although the current is injected as rectangular pulses, with vertical rising and falling edges, the shape of the membrane response to small-amplitude current pulses has a slower rise and fall. For hyperpolarizing and small-amplitude depolarizing current pulses, the rise and fall in the membrane voltage response has an exponential shape because the membrane is responding to the current as would a passive RC circuit. That is, the stimulus causes no change in membrane resistance or capacitance, and thus the time course of the rise and fall simply reflects the time required to discharge or charge the membrane capacitance. Recall that because there is an excess of negative ions inside the axon in comparison to outside, those negative ions will attract some positive ions to the outside of the membrane. These charges remain separated from each other by the cell membrane, similar to the storage of charge in a capacitor. Thus, at least in this passive domain, the membrane response to electrical stimuli closely follows the
same laws that govern an electric circuit composed of a resistor and capacitor connected in parallel.
|
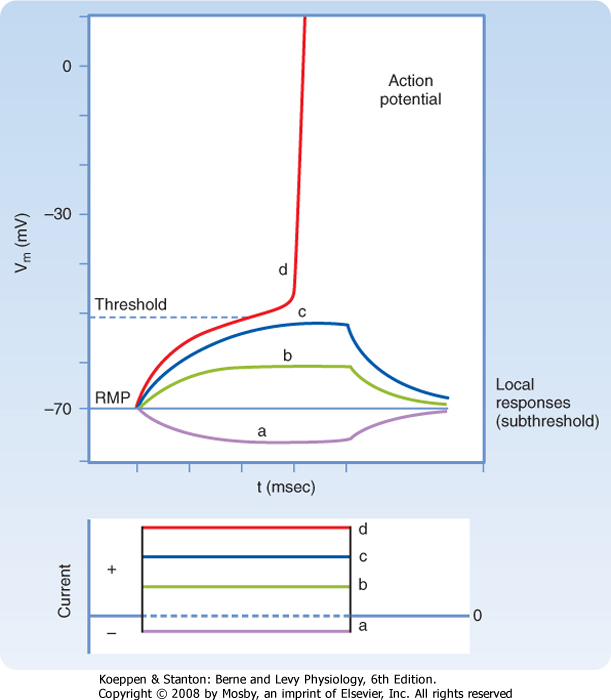
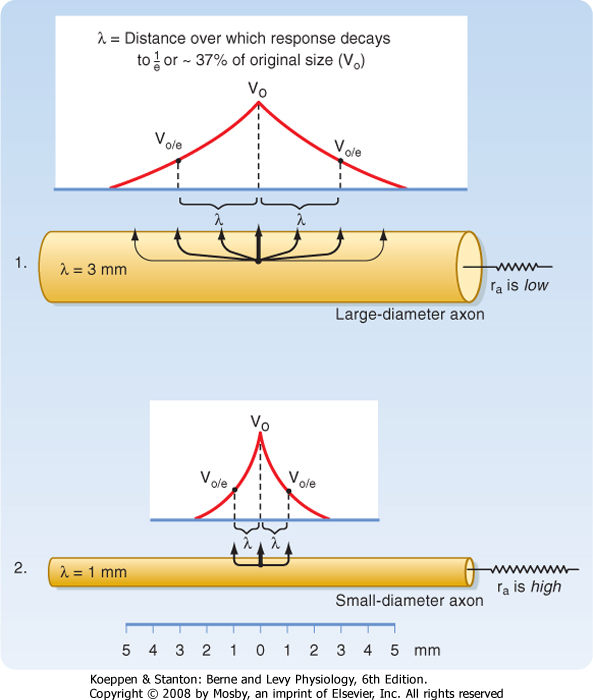
| When current pulses that elicit only passive responses are passed across the plasma membrane, the size of the potential change recorded depends on the distance of the recording electrode from the point of passage of the current (Fig. 5-2). The closer the recording electrode to the site of current passage, the larger and steeper the potential change. The magnitude of the potential change decreases exponentially with distance from the site of passage of the current, and the potential change is said to reflect passive or electrotonic conduction. Such changes do not spread very far along the membrane before they become insignificant. As shown in Figure 5-2, an electrotonically conducted signal dies away over a distance of a few millimeters. The distance over which the potential change decreases to 1/e (37%) of its maximal value is called the length constant or space constant (e is the base of natural logarithms and is equal to 2.7182). A length constant of 1 to 3 mm is typical for mammalian axons.
|
| The length constant can be related to the electrical properties of the axon via cable theory because nerve fibers have many of the properties of an electrical cable. In a perfect cable, the insulation surrounding the core conductor prevents all loss of current to the surrounding medium so that a signal is transmitted along the cable with undiminished strength. If we compare an unmyelinated (see later) nerve fiber with an electrical cable, the plasma membrane equates to the insulation and the cytoplasm to the core conductor, but the plasma membrane is not a perfect insulator. Thus, the spread of signals depends on the ratio of the membrane resistance (rm) and the axial resistance of the axonal cytoplasm (ra). The higher the ratio of rm to ra, the less current lost across the plasma membrane per unit of axonal length, the better the axon can function as a cable, and the longer the distance that a signal can be transmitted electrotonically without significant decrement. A useful analogy is to think of the axon as a garden hose with holes poked in it. The more holes in the hose, the more water will leak out along its length (analogous to more loss of current when rm is low) and the less water delivered to its nozzle.
|
| page 66 |  | | page 67 |
| Figure 5-2 Responses of an axon of a shore crab to a subthreshold rectangular pulse of current recorded intracellularly with an electrode located different distances from the current-passing electrode. As the recording electrode is moved farther from the point of stimulation, the response of the membrane potential is slower and smaller. (Redrawn from Hodgkin AL, Rushton WAH: Proc R Soc B133:97, 1946.) |
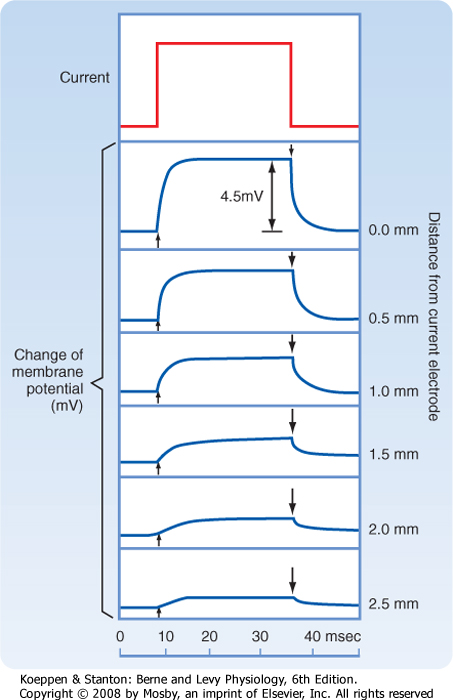
Based on cable theory, the length constant can be related to axonal resistance and is equal to
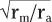 . By using this relationship we can determine how changes in axonal diameter will affect the length constant and hence how the decay of electrotonic potentials will vary. An increase in diameter of the axon will reduce both ra and rm. However, rm is inversely proportional to diameter (because it is related to the circumference of the axon), whereas ra varies inversely to the diameter squared (because it is related to the cross-sectional area of the axon). Thus, ra decreases more rapidly than rm does as axonal
diameter increases, and therefore the length constant increases (Fig. 5-3). . By using this relationship we can determine how changes in axonal diameter will affect the length constant and hence how the decay of electrotonic potentials will vary. An increase in diameter of the axon will reduce both ra and rm. However, rm is inversely proportional to diameter (because it is related to the circumference of the axon), whereas ra varies inversely to the diameter squared (because it is related to the cross-sectional area of the axon). Thus, ra decreases more rapidly than rm does as axonal
diameter increases, and therefore the length constant increases (Fig. 5-3).
|
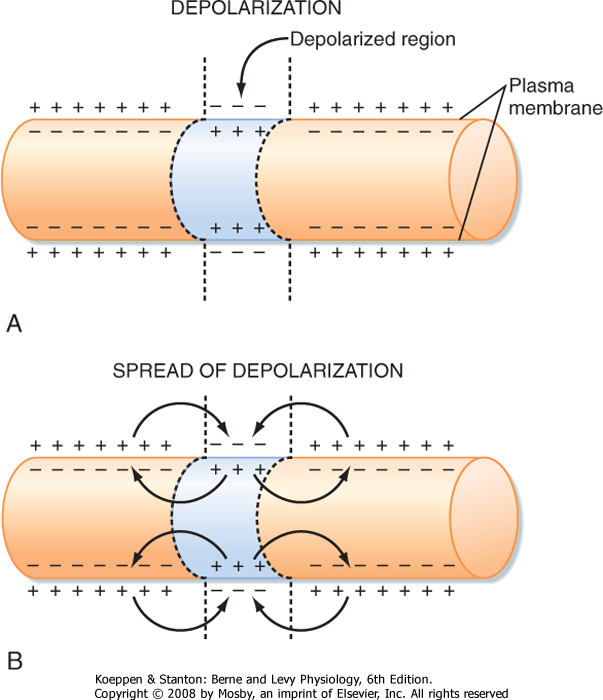
| Membrane capacitance is a major factor that shapes the time course of passive responses. To depolarize an adjacent portion of axon, the injected depolarizing positive charges must draw the inner negative charges from the membrane and thereby free the external positive charges (Fig. 5-4). The time that this process takes increases with the amount of axon membrane to be depolarized.
|
| The Local (Subthreshold) Response
|
| If a somewhat larger depolarizing current pulse is applied to a small portion of the membrane of an axon (Fig. 5-1, c), the voltage response no longer looks like that of a passive RC circuit (e.g., the tail does not decay exponentially). The shape is altered because the stimulus has changed the membrane potential sufficiently to cause the opening of significant numbers of voltage-sensitive Na+ channels (see later). Opening of these channels changes the membrane's resistance and allows Na+ to enter, driven by its electrochemical gradient. This entry of positive charge enhances the depolarization by adding to the current pulse. The resulting depolarization is called a local or subthreshold response. The local response results from active changes in membrane properties (specifically rm), which distinguishes it from the passive electrotonic response. Nevertheless, it is not self-regenerating and does not propagate down the axon but, again, decreases in amplitude with distance. The change in membrane properties is insufficient for what is needed to generate an action potential.
|
| SUPRATHRESHOLD RESPONSE: THE ACTION POTENTIAL
|
| Somewhat larger local responses are observed with still larger depolarizing current pulses until a threshold membrane potential is reached at which a different sort of response, the action potential (or spike), occurs (Fig. 5-5; see also Fig. 5-1, d). For example, the threshold value for the squid giant axon is near -55 mV. When the membrane potential exceeds this value, an action potential is triggered. Thus, the threshold can be defined as the membrane voltage at which there is a 50 : 50 chance of generating an action potential.
|
| The action potential differs from the subthreshold and passive responses in three important ways: (1) it is a much larger response in which the polarity of the membrane potential actually overshoots (the cell interior becomes positive with respect to the exterior), (2) the action potential is propagated down the entire length of the nerve fiber, and (3) the action potential is propagated without decrement (i.e., it maintains its size and shape as it is regenerated along the axon). In addition, when a stimulus even larger than the threshold stimulus is applied, the action potential remains the same and does not increase with greater stimulus strength. A stimulus either produces a full-sized action potential or fails to do so. For this reason, the action potential is described as an all-or-none response.
|
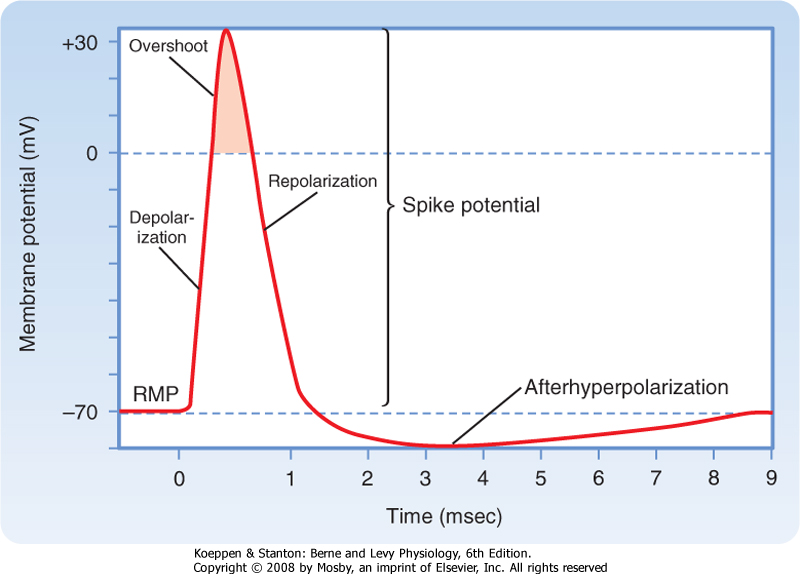
| Action potentials can be generated in other parts of the nerve cell membrane, but their most prominent role is signal conduction along the axon. When the membrane is depolarized to threshold, the depolarization becomes explosive (Fig. 5-5). The depolarization completely depolarizes the membrane and even overshoots such that the membrane potential reverses from negative to positive. The peak of the action potential approaches +50 mV. The membrane potential then returns toward the resting membrane potential almost as rapidly as it was depolarized. After repolarization, a variable hyperpolarization occurs that is known as the afterhyperpolarization. The action potential's depolarization has a duration of 1 to 2 msec, but the hyperpolarizing afterpotential can persist from a few to 100 msec in some cells.
|
| Ionic Basis of Action Potentials
|
| page 67 |  | | page 68 |
| Figure 5-3 Comparison of the length constant, λ, in relation to axon diameter. Note that the increase in diameter is associated with a decrease in ri and an increase in the length constant. (Redrawn from Blankenship J: Neurophysiology. Philadelphia, Mosby, 2002.) |
| Figure 5-4 Mechanism of electrotonic spread of depolarization. A, The reversal of membrane polarity that occurs with local depolarization. B, The local currents that flow to depolarize adjacent areas of the membrane and allow conduction of the depolarization. |
| An action potential is the result of successive, rapid, and transient changes in plasma membrane conductance
to sodium and potassium ions. In the squid giant axon, the resting membrane potential (Vm) is about -70 mV, and the equilibrium potential of K+ (EK) is about -100 mV. An increase in gK would therefore hyperpolarize the membrane, whereas a decrease in gK would tend to depolarize the membrane (see Chapter 2). Conversely, an increase in gNa would cause depolarization and, if of sufficient magnitude, even a reversal in membrane polarity because ENa is about +65 mV in the squid giant axon.
|
| page 68 |  | | page 69 |
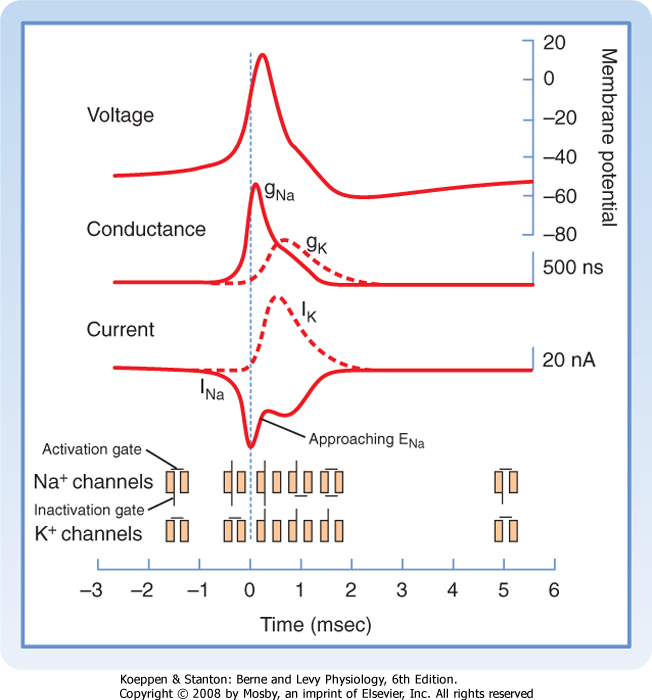
As with the resting membrane potential, the action potential depends on the opposing tendencies of (1) the Na+ gradient to bring the resting membrane potential toward the equilibrium potential for Na+ and (2) the K+ gradient to bring the resting membrane potential toward the equilibrium potential for K+. The relationship between potential, conductance, and ion current during an action potential includes the following (Fig. 5-6):
- A rapid increase in gNa and INa during the early phase of the action potential causes the membrane potential to move toward the equilibrium potential for Na+ (+65 mV). The peak of the action potential does not reach +65 mV because the Na+ channels quickly inactivate, thereby reducing gNa and INa, and because the slower increase in gK and IK provides mounting opposition to depolarization.
- The rapid return of the membrane potential toward the resting potential is caused by the continuing
increase in gK, as well as by the decrease in gNa. The result is that membrane potential is driven toward EK.
- During the hyperpolarizing afterpotential, the membrane potential is actually more negative than the resting potential because gNa has returned to baseline levels but gK remains elevated. Thus, the resting membrane potential is pulled even closer to the K+ equilibrium potential (-100 mV), and the membrane remains hyperpolarized as long as gK remains elevated.
|
|
| Figure 5-5 Components of the action potential with respect to time and voltage. Note that the time scale for the first few milliseconds has been expanded for clarity. RMP, resting membrane potential. (Redrawn from Blankenship J: Neurophysiology. Philadelphia, Mosby, 2002.) |
| Figure 5-6 The action potential and the conductance and currents that underlie the action potential with respect to time. Note that the increased conductance for Na+ (and its inward flow) is associated with the rising phase of the action potential, whereas the slower increase in conductance for K+ (and its outward flow) is associated with repolarization of the membrane and with afterhyperpolarization. The reduction in INa before the peak of the action potential (even though GNa is still high) is due to inactivation of the Na+ channels. (Redrawn from Squires LR et al: Fundamental Neuroscience, 2nd ed. San Diego, CA, Academic Press, 2002.) |
| Early studies on the mechanism underlying action potentials proposed that ion currents pass through separate Na+ and K+ channels, each with distinct characteristics, in the plasma membrane. Subsequent research has supported this interpretation. The amino acid sequences of the channel proteins and many of the functional and structural characteristics of the channels are now known in detail.
|
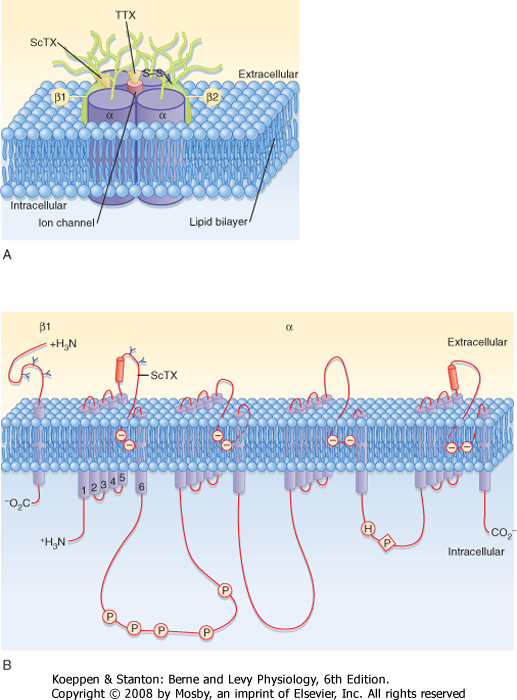
| The structure of a voltage-gated Na+ channel (Fig. 5-7) consists of a single α subunit in association with a β1 and a β2 subunit. The α subunit has four repeated motifs of six transmembrane helices that surround a central ion channel or pore. The channel's walls are partly formed by the number 6 helices in each motif. Most voltage-gated K+ channels consist of only one of the six-helix motifs, but four such subunits are required to form a functional channel. The subunits of one class of voltage-gated K+ channels contain only the number 5 and 6 helices and the intervening pore loop.
|
| page 69 |  | | page 70 |
| Figure 5-7 Three-dimensional model of the voltage-gated Na+ channel. A, The large cylinders represent the 4 α subunits and the two β subunits with the receptor sites for α scorpion toxin (ScTX) and tetrodotoxin (TTX) indicated. B, The β1 subunit and an α subunit are shown with their transmembrane helices. (Redrawn from Squires LR et al: Fundamental Neuroscience, 2nd ed. San Diego, CA, Academic Press, 2002.) |
| Another important characteristic of channels is that in the case of channels underlying the action potential, they are gated by change in voltage (i.e., they are voltage-gated channels). The gates sense the potential across the membrane and then act to either open or close the channel according to the membrane potential. The gates are formed by groups of charged
amino acid residues, and the voltage dependence of the Na+ and K+ channel gates can account for the complex changes in gNa and gK that occur during an action potential.
|
| Behavior of Individual Ion Channels during the Action Potential
|
| page 70 |  | | page 71 |
| Knowledge of the molecular structure of channels has increased our understanding of the basis of their properties. For example, most channels are highly selective for a particular ion. First, by lining the channel walls with either positive or negative charges, one can exclude either cations or anions; however, most channels are also differentially permeable to different ions of the same charge. This further selectivity appears to be the result of requiring ions to become dehydrated as they pass through the narrowest part of a channel, known as the selectivity filter. Ions in solution are hydrated (are surrounded by a shell of H2O molecules), and the radius of this hydration shell is different for each type of ion. In Na+ and K+ channels, to make dehydration energetically possible, negatively polarized amino acid substituents of a particular geometry line the pore of the channel and substitute for the water molecules. Such substitution, however, requires close matching of the filter's size to the ion's hydration shell. Because each ion has a different-sized shell, a particular channel will best allow passage of one particular ionic species. |
 |
| Tetrodotoxin (TTX), one of the most potent poisons known, specifically blocks the Na+ channel. TTX binds to the extracellular side of the sodium channel. Tetraethylammonium (TEA+), another poison, blocks K+ channels. TEA+ enters the K+ channel from the cytoplasmic side and blocks the channel because TEA is unable to pass through it. The ovaries of certain species of puffer fish, also known as blowfish, contain TTX. Raw puffer fish is a highly prized delicacy in Japan. Connoisseurs of puffer fish enjoy the tingling numbness of the lips caused by the minuscule quantities of TTX present in the flesh. Sushi chefs who are trained to remove the ovaries safely are licensed by the government to prepare puffer fish. Despite these precautions, several people die each year as a result of eating improperly prepared puffer fish. |
| Saxitoxin is another blocker of Na+ channels that is produced by the reddish dinoflagellates that are responsible for so-called red tides. Shellfish eat the dinoflagellates and concentrate saxitoxin in their tissues. A person who eats these shellfish may experience life-threatening paralysis within 30 minutes after the meal. |
 |
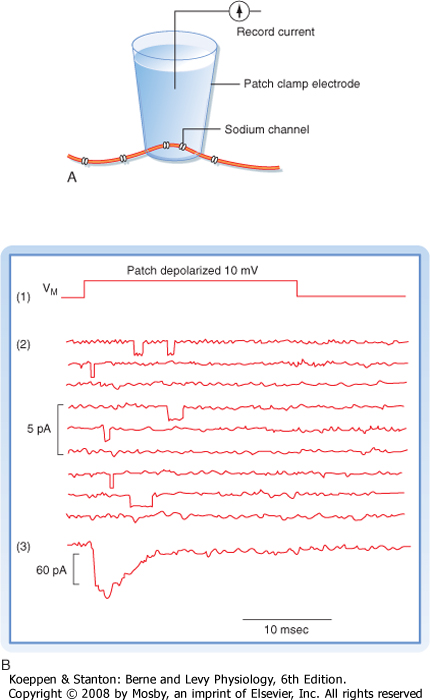
| One way to study the behavior of individual ion channels and how they contribute to the membrane potential is to incorporate either purified ion channel proteins or bits of nerve membrane into planar lipid bilayers separating two aqueous compartments. Electrodes placed in the aqueous compartments can then be used to monitor or impose currents and voltages across the membrane. Another way to study individual ion channels involves the use of patch electrodes. A fire-polished microelectrode is placed against the surface of a cell, and suction is applied to the electrode. A high-resistance seal is formed around the tip of the electrode (Fig. 5-8, A). The sealed patch electrode
can then be used to monitor the activity of whatever channels happen to be trapped inside the seal. Under ideal conditions only one or only a few ion channels of a single type may be present in either the planar membrane or the membrane patch. The ion channels spontaneously oscillate between conductance states: an open state and a closed state. In the case of voltage-gated channels, the time spent in a particular state will be a probabilistic function of the membrane potential.
|
| The action potential starts with a rapid increase in Na+ conductance (gNa; Fig. 5-6). This increase in Na+ conductance reflects the opening of thousands of Na+ channels in response to the depolarization (thus, it is inferred that Na+ channels have a gate that opens in response to depolarization). The open channels allow the influx of Na+ ions, and the effect of this current is to depolarize the membrane further. Note that this is a positive feedback loop that accounts for the explosive nature of the action potential: the Na+ current depolarizes the membrane, which causes more Na+ channels to open, which in turn increases the Na+ current. In sum, the voltage-dependent opening of Na+ channels and the depolarizing action of the Na+ current explain the rising phase of the action potential.
|
| The falling phase of the action potential is the result of two processes: a reduction in gNa and an increase in gK. A decrease in gNa results from repolarizing the membrane because of the voltage dependence of the Na+ channel gate, but if the membrane is experimentally fixed at a depolarized level, Na+ conductance still rapidly drops to zero. This behavior led to the idea that Na+ channels have a second gate, called the inactivation gate, that closes with increasing probability as the membrane is depolarized. In sum, the presence of two gates ensures that a depolarization will always produce a transient increase in gNa (see Fig. 5-6).
|
| When the transient increase in gNa is over, the resting gK (i.e., the leak channels) will allow a current that repolarizes the membrane. In some axons the change in gNa against a fixed gK explains the entire action potential. In many other cases, however, voltage-gated K+ channels also contribute. Voltage-gated K+ channels have only a single gate that opens with depolarization. When the membrane depolarizes during an action potential, many of these K+ channels open, and the result is an increase in gK, which allows a K+ current to flow. The K+ current, opposite the Na+ current, causes repolarization of the membrane. Because voltage-gated K+ channels do not close immediately upon repolarization, the overall membrane conductance to K+ is higher at the end of the action potential than it was just before its initiation. This means that the membrane potential will become closer to the Nernst potential for K+ and is the basis of the afterhyperpolarization that follows a spike. Note that the membrane potential then returns to its original resting value as the voltage-gated K+ channels close. Also note that the K+ channels close as a result of the voltage becoming negative again rather than an inactivation process. Indeed, if one voltage-clamps the membrane at a depolarized level, gK will remain elevated.
|
| page 71 |  | | page 72 |
| Figure 5-8 A, Patch electrode arrangement required to record the ionic currents that flow through the small number of ion channels isolated in the electrode patch. B, Record of (1) a depolarizing voltage pulse applied to patch, (2) multiple records indicating current flow through individual channels, and (3) the summed current response from many trials. (Redrawn from Blankenship J: Neurophysiology. Philadelphia, Mosby, 2002.) |
| Explosive depolarization of the action potential can occur only if a critical number of Na+ channels are recruited. In response to membrane depolarization,
gNa first increases and then, a short time later, decreases. The initial increase in gNa is due to the activation gates of Na+ channels opening in response to the transmembrane voltage. The decrease in gNa is caused by closing of the channels' inactivation gates, which respond more slowly to transmembrane voltage but, once closed, cannot reopen until the membrane is repolarized to near the normal resting membrane potential. Thus, if a cell is partly depolarized, the pool of noninactivated Na+ channels is reduced; consequently, a stimulus may not be able to recruit a sufficient number of Na+ channels to generate an action potential. This is a result of voltage inactivation of some of the Na+ channels.
|
| Accordingly, when a nerve is depolarized slowly, the normal threshold may be passed without an action potential being fired; this phenomenon is called accommodation. Na+ and K+ channels are both involved in accommodation. If the depolarization is slow enough, the critical number of open Na+ channels required to trigger the action potential may never be attained because of inactivation. In addition, K+ channels open slowly in response to the depolarization. The increased gK tends to oppose depolarization of the membrane, thus making it still less likely to fire an action potential.
|
| page 72 |  | | page 73 |
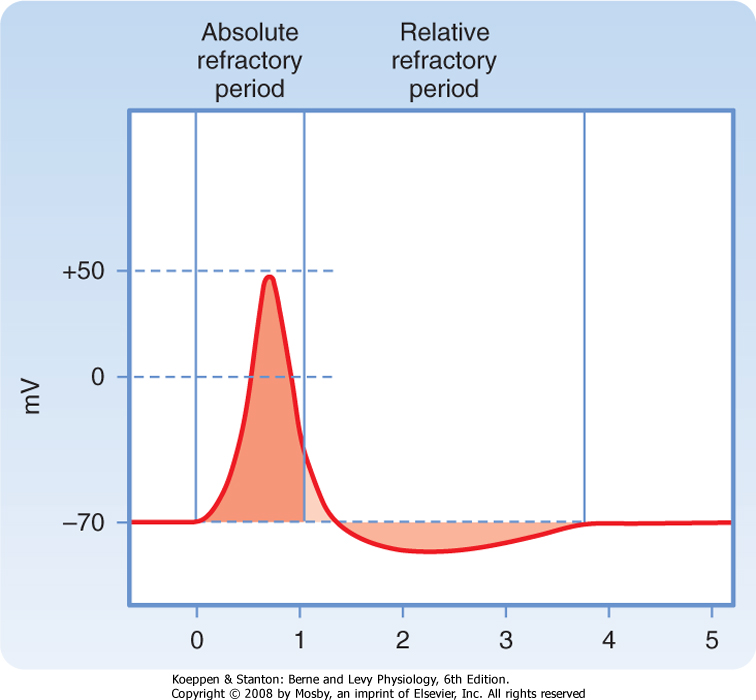
| Figure 5-9 Absolute and relative refractory periods of the action potential. The horizontal scale is in milliseconds. |
| In an inherited disorder called primary hyperkalemic paralysis, patients have episodes of painful spontaneous muscle contractions followed by periods of paralysis of the affected muscles. These symptoms are accompanied by elevated [K+] in plasma and extracellular fluid. Some patients with this disorder have mutations of voltage-gated Na+ channels that result in a decreased rate of voltage inactivation. This results in longer-lasting action potentials in skeletal muscle cells and increased efflux of K+ during each action potential, which can raise extracellular [K+]. |
| The elevation in extracellular [K+] causes depolarization of skeletal muscle cells. Initially, the depolarization brings muscle cells closer to threshold, so spontaneous action potentials and contractions are more likely. As depolarization of the cells becomes more marked, the cells become refractory because of the voltage-inactivated Na+ channels. Consequently, the cells become unable to fire action potentials and are not able to contract in response to action potentials in their motor axons. |
 |
| During much of the action potential the cell is completely refractory to further stimulation. When a cell is refractory, it is unable to fire a second action potential no matter how strongly it is stimulated. This unresponsive state is called the absolute refractory period (Fig. 5-9). The cell is refractory because a large fraction of its Na+ channels are voltage inactivated and cannot be reopened until the membrane is repolarized. In this
state the critical number of Na+ channels required to produce an action potential cannot be recruited.
|
| During the latter part of the action potential, the cell is able to fire a second action potential, but a stronger than normal stimulus is required. This period is called the relative refractory period. Early in the relative refractory period, before the membrane potential has returned to the resting potential level, some Na+ channels are still voltage inactivated. Therefore, a stronger than normal stimulus is required to open the critical number of Na+ channels needed to trigger an action potential. Throughout the relative refractory period, conductance to K+ is elevated, which opposes depolarization of the membrane. This increase in K+ conductance also contributes to the refractoriness and, because of the relatively slow response of the K+ channels, to its extension in time.
|
| CONDUCTION OF ACTION POTENTIALS
|
| A fundamental activity of neurons is to transmit nerve impulses in the form of action potentials. The axons of motor neurons of the ventral horn of the spinal cord conduct action potentials from the cell body of the neuron to skeletal muscle fibers in the body, and the length of the axon may be longer than 1 m.
|
| Conduction of an action potential along an axon is based on local current flow, just as occurs in electrotonic conduction of subthreshold potential changes. Thus, many of the same factors that govern the velocity of electrotonic conduction also determine the speed of propagation of action potentials.
|
| Action Potential as a Self-Reinforcing Signal
|
| Conduction with decrement will not get a signal from one end of an axon to the other unless the axon is very short. For example, in the retina of the eye, the distance from one neuron to the next is so small that electrotonic conduction is sufficient. Axons elsewhere can be up to 1 m or more in length, and therefore most are many times longer than their length constants. For an electrical impulse to travel the full length of these cells with undiminished strength, the action potential regenerates itself as it is conducted along the fiber. The action potential can be said to be propagated, as well as conducted.
|
| Propagation involves the generation of "new" action potentials as they spread along the length of the cell. As seen in Figure 5-4, conduction of the local response occurs via local circuit currents. If instead of a subthreshold local response the instigating stimulus generates an action potential, the explosive depolarization should provide sufficient inward current flow to bring the areas on either side to threshold and generate action potentials. These areas could then provide the local current flow to bring still more distant areas to threshold so that they in turn generate action potentials. In short, propagation involves recurring cycles of depolarization to provide sufficient local current flow for generation of an action potential in an adjacent region of the cell membrane. Thus, the action potential is conducted down the axon, with "new" action potentials being generated along its length. In this way the action potential propagates over long distances while retaining the same size and shape.
|
| page 73 |  | | page 74 |
| Note that as shown in Figure 5-4, the action potential can be generated by a depolarization in the middle of an axon and would be conducted in both directions simultaneously. However, in the nervous system, action potentials are first generated at the initial segment (i.e., where the axon is attached to the neuron
cell body) and conducted to the terminal end. The reason that the initial segment is the first site of generation of an action potential is that it is invested with a high density of voltage-gated Na+ channels, thus giving its membrane the lowest threshold in the cell. In addition, the action potential's refractory periods are also responsible for ensuring that conduction is usually unidirectional. Because the action potential is generated first at the initial segment, any propagating action potential in the middle of the axon cannot generate another in the direction of the cell body since the immediately preceding portions are refractory.
|
| Because the shape and size of the action potential are relatively constant, only variations in the number or frequency of action potentials can be used as the "code" for transmission of information along axons (see later). The maximal frequency is limited by the duration of the absolute and relative refractory periods (Fig. 5-9) and rarely exceeds 1000 spikes per second in large mammalian nerves. This also means that a single axon cannot convey adequate coded information about events that occur more frequently than it can conduct action potentials. For example, signaling of high-frequency sounds may require the combined activity of several neurons.
|
| Effect of Fiber Diameter on Conduction Velocity
|
| In nonmyelinated fibers, conduction velocity is proportional to the square root of the diameter. This effect is related to the longitudinal resistance. As the diameter of a fiber increases, ri decreases with the square of the diameter and rm increases only linearly with diameter. As a result, there is much less resistance to conduction while the membrane is only slightly leakier. This effectively increases the length constant, and thus the action potential will be conducted faster along fibers with large diameters (Fig. 5-3).
|
| However, increasing the diameter also increases the surface area of the plasma membrane over which inner negative and outer positive charges are held to each other. Discharging this increased capacitance tends to slow conduction and mitigate the increase in conduction velocity gained by increasing diameter (Fig. 5-10).
|
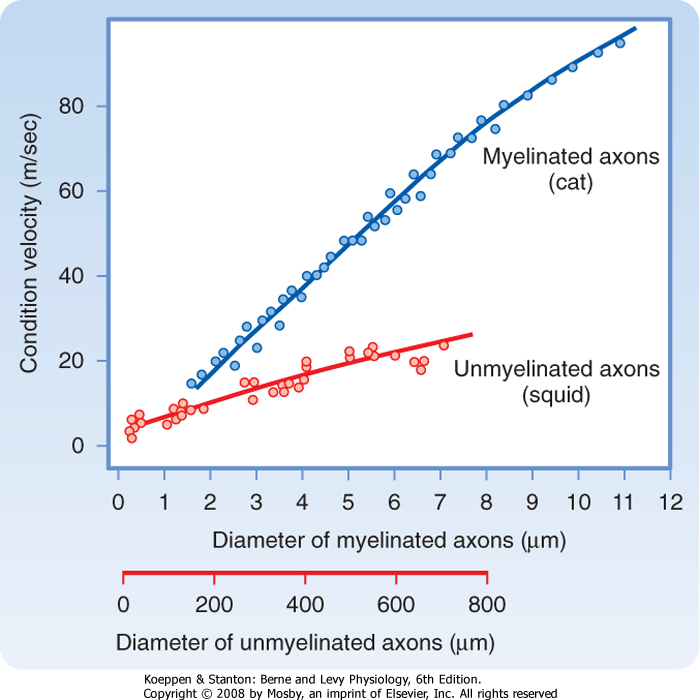
| Figure 5-10 Conduction velocities of myelinated and unmyelinated axons as functions of axon diameter. Myelinated axons are from cat saphenous nerve at 38° C. Unmyelinated axons are from squid at 20° C to 22° C. Note that myelinated axons have greater conduction velocities than unmyelinated axons that are 100 times greater in diameter. (Based on data from Gasser HS, Grundfest H: Am J Physiol 127:393, 1939 [myelinated axons]; and Pumphrey RJ, Young JZ: J Exp Biol 15:453, 1938 [unmyelinated axons].) |
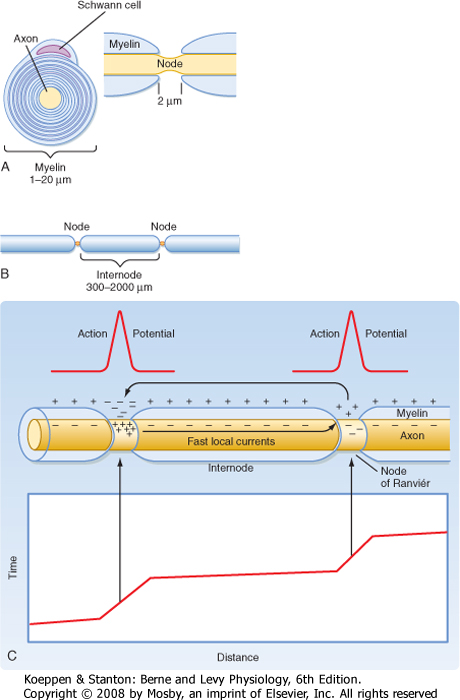
| The speed of conduction in a nerve fiber is determined by the electrical properties of the cytoplasm and the plasma membrane that surrounds the fiber, as well as by its geometry. In vertebrates, many nerve fibers are coated with myelin, and such fibers are said to be myelinated. Myelin consists of the plasma membranes of Schwann cells (located in the peripheral nervous system) or oligodendroglia (located in the central nervous system [CNS]), which wrap around and insulate the nerve fiber (Fig. 5-11, A and B). The myelin sheath consists of several to more than 100 layers of cell plasma membrane. Gaps occur in the myelin sheath every 1 to 2 mm. These gaps are known as nodes of Ranvier and are about 1 μm wide. For all but the smallest diameter axons, a myelinated axon has
much greater conduction velocity than an unmyelinated fiber of the same caliber does because the myelin sheath increases the length constant of the axon, decreases the capacitance of the axon membrane, and restricts the generation of action potentials to the nodes of Ranvier. In short, myelination greatly alters the electrical properties of the axon.
|
| The many wrappings of membrane around the axon increase the effective membrane resistance so that rm/ri and thus the length constant are much greater. This increased membrane resistance means that less of the conducted signal is lost through the membrane and the amplitude of a conducted signal decreases less with distance along the axon.
|
| In addition, the thicker myelin-wrapped membrane enforces a much larger separation between the inside and outside of the axon such that the charges across it are much less tightly bound to each other. Because the effect of membrane capacitance is to slow the rate at which the membrane potential can be changed, this reduced capacitance of myelinated axons means that the depolarization occurs more rapidly. For all these reasons, conduction velocity is greatly increased by myelination, and the current generated at one node of Ranvier is conducted at great speed to the next (Fig. 5-12).
|
| page 74 |  | | page 75 |
| Figure 5-11 A, Schematic drawings, in cross section and longitudinal section through a node of Ranvier, of a Schwann cell wrapped around an axon to form myelin. Note that the axon is exposed to the extracellular space only at the node of Ranvier. B, View of two nodes and the intervening internode of myelin. (Redrawn from Squires LR et al: Fundamental Neuroscience, 2nd ed. San Diego, CA, Academic Press, 2002.) C, Saltatory conduction in a myelinated axon with a plot of the action potential location along the axon vs. time. Note the short time taken for the action potential to traverse the large distance between nodes (shallow sloped lines on the plot) due to the high resistance and low capacitance of the internodal region. In contrast, the action potential slows as it crosses each node (steep sloped line segments). (Redrawn from Blankenship J: Neurophysiology. Philadelphia, Mosby, 2002.) |
| The Na+ channels that bring about generation of the action potential are highly concentrated at the nodes of Ranvier and are not found between them. Thus, the
action potential is regenerated only at the nodes of Ranvier (1 to 2 mm apart) rather than being regenerated continuously along the fiber, as is the case in an unmyelinated fiber. Resistance to the flow of ions across the many layers that make up the myelin sheath is so high that transmembrane currents are effectively restricted to the short stretches of naked plasma membrane that occur at the nodes of Ranvier (Fig. 5-11, C). Therefore, the action potential is regenerated at each successive node. The local currents entering the node are almost entirely conducted from one node to the next node, bringing it to threshold in about 20 μsec! Thus, the action potential appears to "jump" from one node of Ranvier to the next, and the process is called saltatory (from the Latin word saltare, to leap) conduction.
|
| Functional Consequences of Myelination
|
| page 75 |  | | page 76 |
| Figure 5-12 Comparison of action potential conduction in an unmyelinated axon and a myelinated axon. At the initial time (A and C), an action potential is being generated at the left side of each axon. Note that the inward current in the unmyelinated axon (A) is depolarizing an adjacent portion, whereas the inward current in the myelinated axon (C) is depolarizing the next node. At the second instant in time (B and D), the action potential in the unmyelinated axon has been generated in the adjacent portion while the action potential in the myelinated axon (D) has been generated at subsequent nodes and is already depolarizing the last node to the right. (Redrawn from Castro A et al: Neuroscience: An Outline Approach. Philadelphia, Mosby, 2002.) |
| In some diseases known as demyelinating disorders, the myelin sheath deteriorates. In multiple sclerosis, scattered progressive demyelination of axons in the CNS results in loss of motor control. The neuropathy common in severe cases of diabetes mellitus is caused by the demyelination of peripheral axons. When myelin is lost, the length constant, which is dramatically increased by myelination, becomes much shorter. Hence, the action potential loses amplitude as it is electrotonically conducted from one node of Ranvier to the next. If demyelination is sufficiently severe, the action potential may arrive at the next node of Ranvier with insufficient strength to fire an action potential. The axon may then fail to propagate action potentials. |
 |
| Although our nerve fibers are much smaller in diameter than squid giant axons, our axons conduct at comparable or even faster speeds because of myelination. The unmyelinated squid giant axon has a 500-μm diameter, which results in it having a conduction velocity of about 20 m/sec (Fig. 5-10). However, unmyelinated mammalian nerve fibers, which have diameters under
2 μm have conduction velocities less than 2 m/sec. With such slow conduction velocity, reflex withdrawal of the foot from a sharp object would take at least 2 seconds as the information is conducted from foot to spinal cord by this axon and the withdrawal command conducted back to the muscles. The myelin sheath that surrounds many mammalian nerve fibers is responsible for the greatly increased conduction velocity over that of unmyelinated fibers of similar diameter. A 10-μm myelinated fiber would have a conduction velocity in the range of 50 m/sec, more than twice that of the 500-μm squid giant axon. The high conduction velocity permits reflexes that are fast and also supports efficient and complex mental processing.
|
| The action potentials of myelinated axons do not have a hyperpolarizing afterpotential or extended relative refractory period because they lack K+ channels at their nodes. This increases the rate at which these fast-conducting axons can fire. Myelinated axons are also more metabolically efficient than unmyelinated axons. Na+,K+-ATPase extrudes the Na+ that enters and reaccumulates the K+ that leaves the cell during action potentials. In myelinated axons, ionic currents are restricted to the small fraction of the membrane surface at the nodes of Ranvier. For this reason, far fewer ions traverse a unit length of fiber membrane, and much less ion pumping-and energy expenditure-is required to maintain the gradients.
|
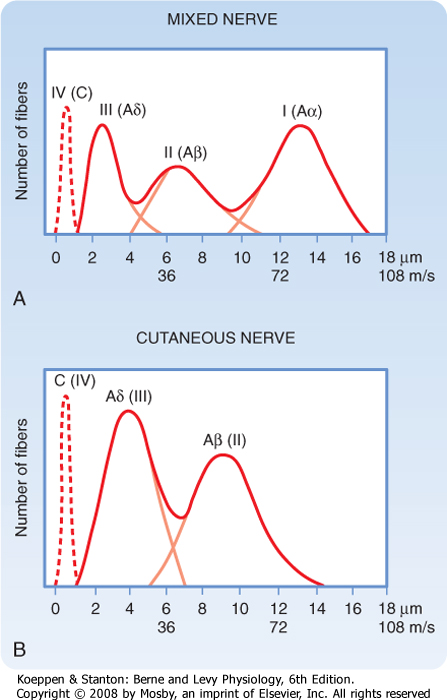
| An action potential can be recorded with a microelectode without penetrating the axon by placing two spaced electrodes on its surface and comparing the electrical charge at each point. An electrode located where there is an action potential would be somewhat negative in comparison to an electrode where there is no action potential (Fig. 5-12). As the action potential is conducted to the second electrode, the polarity of the recording reverses. This technique is used clinically to assess nerve function. Peripheral nerves and many central pathways consist of a population of axons of various diameter, some of which are myelinated and some are not. Consequently, action potentials travel at different velocities in the individual axons. As a result, a recording from such a nerve with external electrodes does not show a single synchronous peak but a series of peaks that vary in time (reflecting the conduction velocity of groups of axons) and in size (reflecting the number of axons in each velocity group). This is called a compound action potential, and its particular form is somewhat characteristic for each nerve's particular axon population (Fig 5-13). The clinical value of such a recording is its ability, in certain disease states, to reveal the dysfunction of a particular group of axons associated with specific functions, as well as the noninvasive nature of the technique because it can be done with skin surface electrodes (Table 5-1). |
 |
| page 76 |  | | page 77 |
|
Table 5-1.
Correlation of Axon Groups, as Revealed by Compound Action Potential Recordings, with Their Functional Properties |
| Electrophysiologic Classification of Peripheral Nerves | Classification of Afferent Fibers ONLY (Class/Group) | Fiber Diameter (mm) | Conduction Velocity (m/sec) | Receptor Supplied |
| Sensory Fiber Type |
| Aα | Ia and Ib | 0.13-20 | 0.80-120 | Primary muscle spindles, Golgi tendon organ |
| Aβ | II | 0.16-12 | 0.35-75 | Secondary muscle spindles, skin mechanoreceptors |
| Aδ | III | 0.11-51 | 0.15-30 | Skin mechanoreceptors, thermal receptors, nociceptors |
| C | IV | 0.2-1.5 | 0.5-2 | Skin mechanoreceptors, thermal receptors, nociceptors |
| Motor Fiber Type |
| Aα | N/A | 0.12-20 | 0.72-120 | Extrafusal skeletal muscle fibers |
| Aγ | N/A | 0.12-8.2 | 0.12-48 | Intrafusal muscle fibers |
| B | N/A | 0.21-33 | 0.86-18 | Preganglionic autonomic fibers |
| C | N/A | 0.2-2 | 0.5-2 | Postganglionic autonomic fibers |
|
From Haines DE (ed): Fundamental Neuroscience for Basic and Clinical Applications, 3rd ed. Philadelphia, Churchill Livingstone, 2006.
|
| Figure 5-13 The compound action potential evoked in a mixed nerve (A) and a cutaneous nerve (B) in response to electrical stimulation. Note the increased number of small-diameter fibers and the absence of Aα fibers in the cutaneous nerve. (From Haines DE [ed]: Fundamental Neuroscience for Basic and Clinical Applications, 3rd ed. Philadelphia, Churchill Livingstone, 2006.) |
| SENSORY TRANSDUCTION AND CODING
|
| As already discussed, the mechanism for the generation of action potentials is depolarization of the initial segment of the axon. However, for the nervous system to receive input, it must be stimulated by the application of energy, and this energy must be transduced into a neural event (i.e., the action potential discussed earlier). The parameters of the energy (e.g., its intensity and duration) are then encoded into patterns of action potentials conducted over one or more axons.
|
| Stimulation is the action of environmental energy through activation of one or more sensory receptors. A stimulus is the environmental event that excites sensory receptors, which then provide information about the stimulus to the CNS. The response to the stimulus is the effect that the stimulus has on the organism. Responses can be recognized at several levels, including (1) receptor potentials in the sensory receptors; (2) transmission of action potentials along axons in sensory pathways; (3) synaptic events in central neural networks; and (4) motor activity triggered by sensory stimulation, which is ultimately observed as behavior. The process that enables a sensory receptor to respond usefully to a stimulus is called sensory transduction.
|
| Environmental events that involve sensory transduction can be mechanical, thermal, chemical, or other forms of energy; the type of transduction depends on the sensory apparatus that serves as a transducer. Although humans cannot sense electrical or magnetic fields, other animals can respond to such stimuli. For example, many fish have electroreceptors, and various fish and birds use the earth's magnetic field to orient themselves during migration.
|
| page 77 |  | | page 78 |
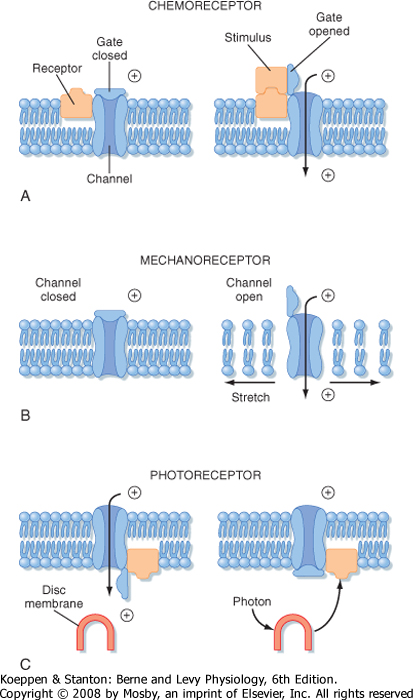
| Figure 5-14 Conceptual models of transducer mechanisms in three types of receptors. A, Chemoreceptor; B, mechanore-ceptor; C, photoreceptor. |
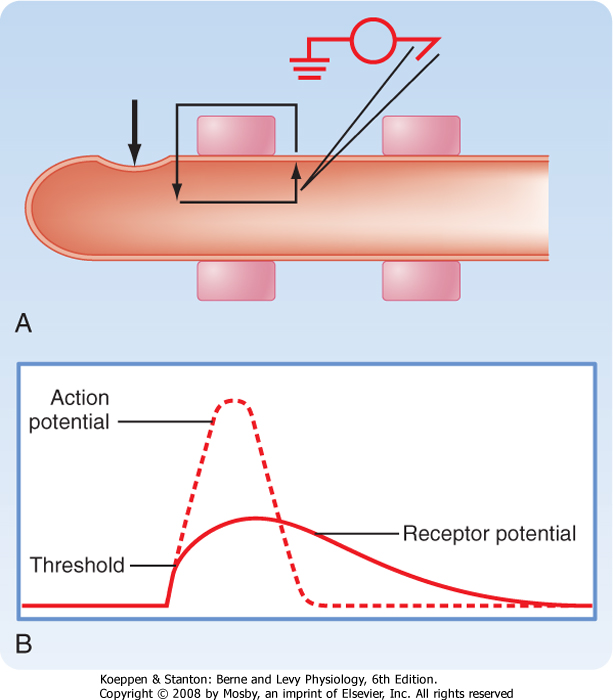
| Figure 5-15 A, Current flow (small arrows) produced by stimulation of a mechanoreceptor at the site indicated by the large arrow. An intracellular recording electrode is placed at the first node of Ranvier. B, The receptor potential produced by the current and an action potential that would be superimposed on the receptor potential if it exceeds threshold at the first node of Ranvier. |
| Figure 5-14 shows three examples of how stimuli can alter the membrane properties of the specific sensory receptor neurons that transduce such stimuli (details for each of these examples are found in other chapters). Figure 5-14, A, illustrates how a chemoreceptor, such as we use for taste and smell, might respond when a chemical stimulant reacts with receptor
molecules on the plasma membrane of the sensory receptor. (Note the distinction between a sensory receptor, which includes one or more cells, and a receptor molecule, which is a protein inserted into the membrane of a cell.) Binding of the chemical stimulant to the receptor molecule opens an ion channel, which enables the influx of an ionic current that depolarizes the sensory receptor cell. (This is similar to what is described for ligand-gated channels in Chapter 6.) In Figure 5-14, B, the ion channel of a mechanoreceptor, such as found in the skin, opens in response to the application of a mechanical force along the membrane, and this allows an influx of current to depolarize the sensory receptor. In Figure 5-14, C, the ion channel of a retinal photoreceptor cell (so called because it responds to light) is open in the dark and closed when
a photon is absorbed by pigment on an internal disc membrane. In this case, an influx of current occurs in the dark; the current ceases when light is applied. When the current stops, the photoreceptor hyperpolarizes. (Because capture of the photon is distant from the ion channel that it influences, this process must involve a "second messenger" mechanism.)
|
| page 78 |  | | page 79 |
| Sensory transduction generally produces a receptor potential in the primary afferent neuron. The receptor potential is usually a depolarizing event that results from inward current flow, which brings the membrane potential of the sensory receptor toward the threshold needed to trigger an action potential, as explained earlier. For example, a mechanical stimulus, such as pressure on the skin of a finger, can distort the membrane of a mechanoreceptor, as shown in Figure 5-15, A. This distortion causes inward current flow at the end of the axon and longitudinal and outward current flow along the axon. The outward current produces a depolarization (the receptor potential) that might exceed the threshold for an action potential (Fig. 5-15, B). If so, the action potential will travel along this primary afferent fiber to the CNS and signal sensory information. There can be variations on this theme in which the primary afferent fiber terminates on a separate, peripherally located sensory receptor cell. For example, in the cochlea, primary afferent fibers end on hair cells. Sensory transduction in such sense organs is made more complex by this arrangement. In photoreceptors, moreover, the receptor
potential is hyperpolarizing, as mentioned earlier, and interruption of the dark current is the signal event. Information about each of these mechanisms is discussed in Chapter 8.
|
| A stimulus threshold is the weakest stimulus that can be reliably detected. For detection, a stimulus must produce receptor potentials that are large enough to activate one or more primary afferent fibers. Weaker intensities of stimulation can produce subthreshold receptor potentials; however, such stimuli would not excite central sensory neurons and thus could not be detected. Furthermore, the number of primary afferent neurons that need to be excited for sensory detection depends on the requirements for spatial and temporal summation in the pathway (see Chapter 6).
|
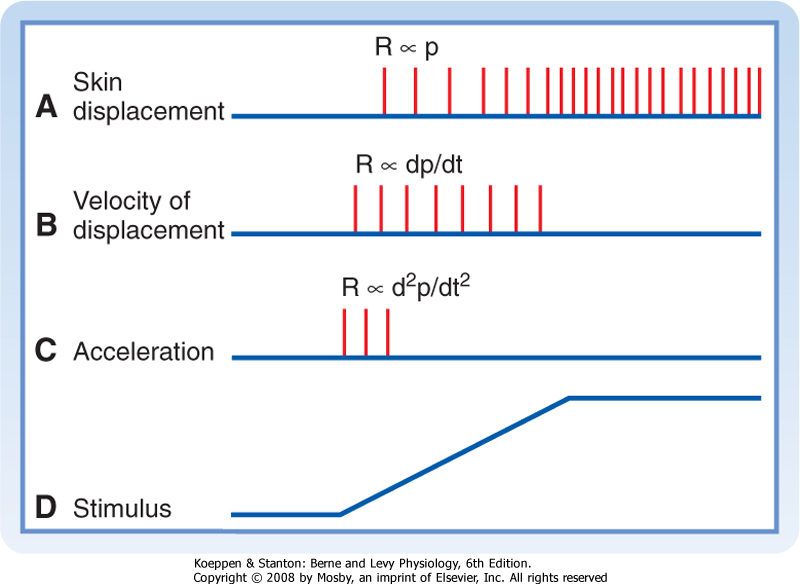
| Adaptation, a change in the way that a receptor responds to sequential or prolonged stimulation, is a characteristic property of sensory receptors that makes them better suited to signal particular kinds of sensory information. For example, slowly adapting receptors in the skin produce a repetitive discharge in response to a prolonged stimulus. However, rapidly adapting receptors produce only a few spikes at the onset (or offset) of the same stimulus. Figure 5-16 shows the responses of three types of receptors to slow deflection of the skin graphed at the bottom. The functional implication is that different temporal features of a stimulus can be signaled by receptors with different adaptation rates.
|
| Figure 5-16 Responses of slowly and rapidly adapting mechanoreceptors to displacement of the skin. A to C are the discharges of primary afferent fibers during a ramp-and-hold stimulus shown in D. A shows the response of a slowly adapting receptor that signals the magnitude and duration of displacement. B shows the response of a rapidly adapting receptor whose output signals the velocity of displacement. C shows the response of a different rapidly adapting receptor that responds to acceleration. |
| The relationship between the location of a stimulus and activation of particular sensory neurons is a major
theme in sensory physiology. The receptive field of a sensory neuron is the region that when stimulated, affects the behavior of the neuron. For example, a sensory receptor might be activated by indentation of only a small area of skin. That area is the excitatory receptive field of the sensory receptor. A neuron in the CNS might be excited by stimulation of a receptive field several times as large as that of a sensory receptor because it may receive information from many sensory receptors, each with a slightly different receptive field. The receptive field of that CNS neuron is the sum of the receptive fields of the sensory receptors that influence it. The location of the receptive field is determined by the location of the sensory transduction apparatus responsible for signaling information about the stimulus to the sensory neuron.
|
| Generally, the receptive fields of sensory receptors are excitatory. However, a central sensory neuron can have either an excitatory or an inhibitory receptive field or, indeed, a complex receptive field that includes areas that excite it and areas that inhibit it. Examples of such complex receptive fields will be discussed in Chapters 7 and 8.
|
| Sensory neurons encode stimuli. In the process of sensory transduction, one or more aspects of the stimulus must be encoded in a way that can be interpreted by the CNS. The encoded information is an abstraction based on (1) which sensory receptors are activated, (2) the responses of sensory receptors to the stimulus, and (3) information processing in the sensory pathway. Some of the aspects of stimuli that may be encoded include the sensory modality, spatial location, threshold, intensity, frequency, and duration. Other aspects of stimuli that are encoded are presented in relation to particular sensory systems in later chapters.
|
| A sensory modality is a readily identified class of sensation. For example, sustained mechanical stimuli applied to the skin result in sensations of touch or pressure, and transient mechanical stimuli may evoke sensations of flutter or vibration. Other cutaneous modalities include cold, warmth, and pain. Vision, audition, position, taste, and smell are examples of noncutaneous sensory modalities. The encoding of sensory modality is done by labeled-line sensory channels in most sensory systems and derives from the specific sensory receptors at its beginning. For example, the visual pathway includes photoreceptors, neurons in the retina, the lateral geniculate nucleus of the thalamus, and the visual areas of the cerebral cortex (see Chapter 8). The normal means of activating the visual system is light striking the retina. However, mechanical (e.g., by pressure on the eyeball) or electrical stimulation of neurons in the visual pathway also produces a visual sensation. Thus, neurons of the visual system can be regarded as a labeled line, which when activated by whatever means, causes a visual sensation.
|
| page 79 |  | | page 80 |
| The spatial location of a stimulus is signaled by activation of the particular population of sensory
neurons whose receptive fields are affected by the stimulus. The information may be encoded in the CNS by a neural map. For example, a somatotopic map is formed by arrays of neurons in the somatosensory cortex that receive information from corresponding locations on the body surface (see Chapter 7). In the visual system, points on the retina are represented by neuronal arrays that form retinotopic maps (see Chapter 8). In the auditory system the frequency of sounds is represented in tonotopic maps (see Chapter 8). In some cases an inhibitory receptive field or a contrasting border between an excitatory and an inhibitory receptive field can have localizing value. Resolution of two different adjacent stimuli may depend on the excitation of partially separate populations of neurons and on inhibitory interactions.
|
| Stimulus intensity may be encoded in a number of ways. Because action potentials have a uniform magnitude, some sensory neurons encode intensity by their frequency of discharge. The relationship between stimulus intensity and response can be plotted as a stimulus-response function. For many sensory neurons, the stimulus-response function approximates an exponential curve with an exponent less than, equal to, or greater than 1. Stimulus-response functions with fractional exponents characterize many mechanoreceptors. Thermoreceptors, which detect changes in temperature, have linear stimulus-response curves (exponent of 1). Nociceptors, which detect painful stimuli, may have linear or positively accelerating stimulus-response functions (i.e., the exponent for these curves is 1 or greater). The positively accelerating stimulus-response functions of nociceptors help explain the urgency that is experienced as the pain sensation increases.
|
| Another way in which stimulus intensity is encoded is by the number of sensory receptors that are activated. A stimulus at the threshold for perception may activate just one or just a few primary afferent neurons of an appropriate class, whereas a strong stimulus of the same type may excite many similar receptors. Central sensory neurons that receive input from this particular class of sensory receptor would be more powerfully activated as more primary afferent neurons discharge. Greater activity in central sensory neurons is perceived as a stronger stimulus.
|
| Stimuli of different intensities may also activate different sets of sensory receptors. For example, a weak mechanical stimulus applied to the skin might activate only mechanoreceptors, whereas a strong mechanical stimulus might activate both mechanoreceptors and nociceptors. In this case the sensation evoked by the stronger stimulus would be more intense, and the quality perceived would be different.
|
| Stimulus frequency can sometimes be encoded by action potentials whose interspike intervals correspond exactly to the intervals between stimuli (e.g., at intervals corresponding to that of a low-frequency vibration). In other cases, a given neuron may discharge at intervals that are multiples of the interstimulus interval. Clearly, a discharge rate cannot unambiguously signal both frequency and intensity in the same system.
|
| Another method for encoding information is to encode the communicated information into structured patterns of nerve impulse trains. Several different types of nerve impulse codes have been proposed. A commonly used code depends on the mean discharge frequency. For example, in many sensory systems, increases in the intensity of a stimulus cause a greater frequency of discharge of the sensory neurons. Other candidate codes depend on the time of firing, the temporal pattern, and the duration of bursts.
|
| Stimulus duration may be encoded in slowly adapting sensory neurons by the duration of enhanced firing. The beginning and end of a stimulus may be signaled by transient discharges of rapidly adapting sensory receptors (Fig. 5-16).
|
| page 80 |  | | page 81 |
- The action potential is generated by the rapid opening and subsequent voltage inactivation of voltage-dependent Na+ channels and the delayed opening and closing of voltage-dependent K+ channels.
- Ion channels are integral membrane proteins that have ion-selective pores. Different regions of an ion channel protein act as gates to activate and inactivate the channel. An ion channel typically has two states: high conductance (open) and zero conductance (closed). The channel oscillates randomly between the open and closed states. For a voltage-dependent channel, the fraction of time that the channel spends in the open state is a function of the transmembrane potential difference.
- Local circuit currents produce electrotonic conduction. Both subthreshold signals and action potentials are conducted along the length of a cell by local circuit currents. The action potential is propagated rather than merely being conducted; it is regenerated as it moves along the axon. In this way an action potential remains the same size and shape as it is conducted.
- Voltage inactivation of Na+ channels and membrane hyperpolarization due to slow closure of K+ channels are the major factors determining the absolute and relative refractory periods that limit the maximum firing rate of action potentials.
- The velocity of conduction is determined by the electrical properties of the axon. A large-diameter axon has faster conduction velocity.
- Myelination dramatically increases the conduction velocity of a nerve axon. Because myelin increases membrane resistance and lowers membrane capacitance, an action potential is conducted very rapidly from one node of Ranvier to the next. Since it takes much longer to generate an action potential at each node than it does for the action potential to be
conducted between nodes, the action potential appears to jump from node to node; this form of conduction is called saltatory conduction.
- Receptor potentials are changes in membrane potential that result from transduction of a sensory stimulus. Receptor adaptation is a mechanism for signaling the temporal features of a stimulus.
- The receptive field of a receptor or any central neuron is that area of the periphery that affects its activity. The specific type of energy that stimulates a response in the receptor cell defines the modality of the sensory pathway. Timing, duration, and patterns of action potentials encode stimulus intensity, frequency, and duration.
|
 |
|


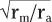 . By using this relationship we can determine how changes in axonal diameter will affect the length constant and hence how the decay of electrotonic potentials will vary. An increase in diameter of the axon will reduce both ra and rm. However, rm is inversely proportional to diameter (because it is related to the circumference of the axon), whereas ra varies inversely to the diameter squared (because it is related to the cross-sectional area of the axon). Thus, ra decreases more rapidly than rm does as axonal
diameter increases, and therefore the length constant increases (
. By using this relationship we can determine how changes in axonal diameter will affect the length constant and hence how the decay of electrotonic potentials will vary. An increase in diameter of the axon will reduce both ra and rm. However, rm is inversely proportional to diameter (because it is related to the circumference of the axon), whereas ra varies inversely to the diameter squared (because it is related to the cross-sectional area of the axon). Thus, ra decreases more rapidly than rm does as axonal
diameter increases, and therefore the length constant increases (